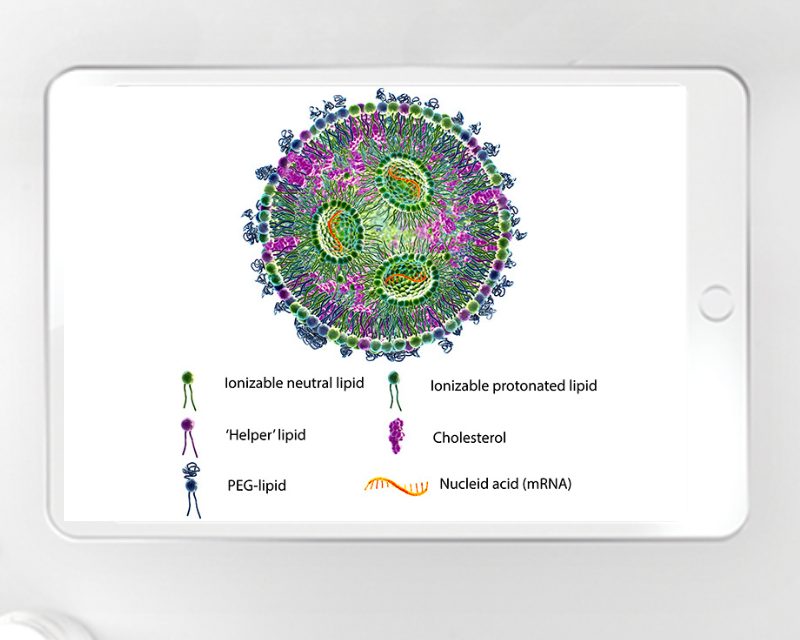
Supporting the pharmaceutical industry – how a CDMO can help
After their recent webinar, European Pharmaceutical Review’s Head of Content Ian Betteridge spoke with the team at Adragos Pharma to discuss the role of a CDMO and their importance in the fill and finish sector.