Improvements in skin and nail drug delivery using nano formulations
Posted: 26 August 2021 | Dave Cook (Blueberry Therapeutics), Dave Edwards (Blueberry Therapeutics) | No comments yet
It might be easy to dismiss non-life-threatening diseases of the skin or nail as trivial, but their impact on physical and mental wellbeing can be significant. Such diseases are obvious targets for topical treatments, but the lack of effective formulations means patients are often faced with the prospect of oral medication – sometimes with troubling side effects. New developments in the field of nanomedicines could hold the key to improving patient wellbeing worldwide. Here, Dave Edwards and Dave Cook discuss the challenges of treating skin and nail diseases, and how nanotechnology could be the future of dermal drug delivery.

WE WILL ALL have encountered someone dealing with or who has previously experienced a common ailment of the skin or nails, such as eczema (atopic dermatitis), acne or a fungal nail infection. For people suffering with these everyday conditions, managing them can have a significant impact on their life – not just because of the physical symptoms, which can be painful, but because they affect the ‘physical self’ we present to the world, impacting patients’ confidence, self‑esteem and even mental health.
Our skin and nails have evolved as effective protective barriers to the world at large, but this also makes them challenging targets when it comes to formulating effective topical treatments for these everyday illnesses. Now nanomedicine – the application of nanotechnology to pharmaceutical formulations – is offering new opportunities for topical drug development, formulation and delivery, which may limit the need for oral treatments and provide a light at the end of the tunnel for patients.
The benefits of topical remedies
Topical drugs, if formulated correctly, represent a non-invasive, painless and convenient alternative to other routes such as injections or oral drug delivery. For common skin and nail diseases and disorders, such as fungal infections, psoriasis, skin cancers, dermatitis and acne, direct treatment to the skin would logically be the best route for administration – targeting the site of disease while limiting any unwanted systemic exposure and associated toxicities.
Beyond the skin, exciting opportunities may result from enhanced transdermal delivery into subcutaneous tissues and then into the systemic circulation. There is the potential to deliver steady and sustained drug levels over prolonged time periods, without the peaks and troughs in drug plasma concentrations that are common in more acute dosing routes, including oral administration.
The challenge of skin and nail-based delivery
The skin has evolved to be an excellent natural barrier,1 meaning when it comes to drug administration, it remains a challenge for pharmaceutical development. Due to its multi‑layered composition, the capacity for a drug to enter and pass through our bodies safely relies upon its ability to penetrate both the hydrophobic and hydrophilic layers of the skin, which many existing formulations are not designed to do. There are also physiological factors that can affect skin permeability – from a person’s age and gender, to underlying skin disorders.2-3
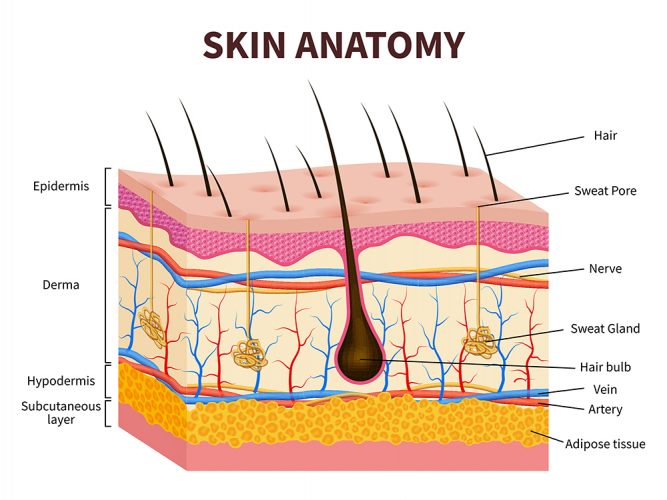

Figure 1: Anatomical structure of the layers of the skin.
The nail too presents an obstacle to effective therapeutic intervention. For example, in order to reach the nail bed – the site of nail infections – a topical medicine must penetrate the nail plate, a hardened and effective barrier. Hydrophilic properties of the nail plate also impede the penetration of topical (often hydrophobic) drugs. These factors alongside the sequestration of pathogens between the nail bed and plate, and slow nail growth rate, make eliminating infections challenging.4
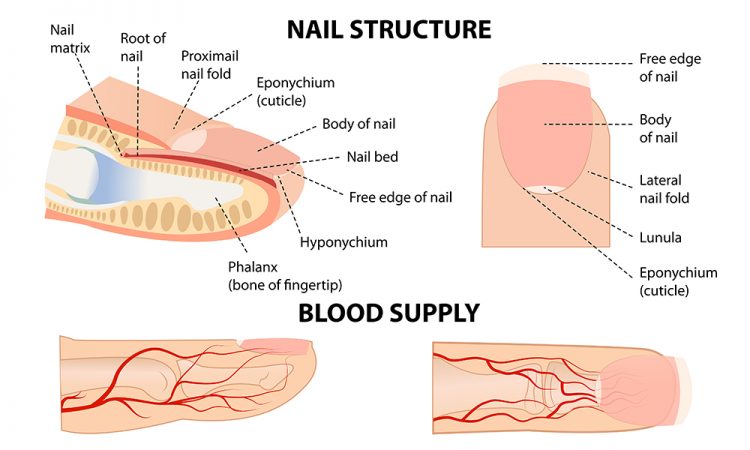

Figure 2: Anatomical structure of the nail.
Applying nanotechnology to enhance delivery
Nanotechnology is defined as science, engineering and technology conducted at the nanoscale (about one to 100 nanometres) and may provide a means to overcome the barrier functions that limit current topical drug delivery formulations.
The most common nanoparticle types best suited for topical nanomedicine development are:
- Liposomes: an aqueous centre where drugs are surrounded by hydrophobic membrane in the form of a lipid bilayer
- Dendrimer polymers: particles within which active compounds can be trapped or absorbed into a polymeric core
- Micelles: ultramicroscopic globular structures with excellent biocompatibility that can be formed in either aqueous or non-aqueous solutions.
Nanomedicine formulations have shown to be promising drug delivery systems, aimed at increasing efficacy as controlled active ingredient delivery systems and eliminating low biodistribution and efficacy, as well as toxicity associated with traditionally formulated medications.
Encapsulation of unstable active ingredients in nano-sized drug delivery systems can increase their stability as well as enhance the solubility of both lipophilic and hydrophilic active ingredients.
If enhanced skin and nail penetration can be achieved through applying nanotechnology, there are potential opportunities to reduce dose, treatment times and levels of systemic toxicity. This would ultimately improve patient compliance, resulting in unprecedented solutions for the treatment of skin and nail conditions.
Three disease areas where topical nanomedicines are proving effective are atopic dermatitis, acne and fungal nail infection.
Atopic dermatitis

Atopic dermatitis is a chronic, relapsing inflammatory skin disease characterised by acute flare ups of lesions over dry skin. Despite the approval of novel injectable treatments that target inflammation, there remains a need for better topical treatments with improved skin bioavailability that reduce inflammation, alleviate itching and restore barrier function, without eliciting the known toxicities of existing treatments, such as skin atrophy and pain at application site.
The application of nanomedicines including nanoparticles, liposomes, nano-gels, nano-mixtures, nano-emulsions and other nano-carriers is being actively investigated for atopic dermatitis.5-6
These novel delivery systems are designed to improve formulation stability, modify permeation and penetration into the skin, and extend the retention or release of active drugs within the skin. Silver, silver-lipid, cyclosporin A, tacrolimus, hydrocortisone and antimicrobial hydroxytyrosol nanoparticles and nanoemulsions have been observed to have optimal rheological and antimicrobial properties, enhanced skin delivery to the deepest layers and fewer adverse events.
Acne
Acne vulgaris is a highly prevalent and chronic inflammatory skin disorder that affects a large proportion of teenagers (approximately 85 percent) and causes serious negative impacts on the social and psychological wellbeing of individuals.7
Nanotechnologies can help to tackle many of the challenges faced by the pharmaceutical industry when it comes to skin and nail drug delivery”
Many topical medications for acne cause side effects such as skin irritation, erythema, eczematous irritation, skin peeling, dryness and photosensitivity, leading to issues with patient compliance. As a result, nanomedicine-based formulations have been developed for topical acne treatment to overcome these difficulties.
Lipid nanoparticles, nanoemulsions and vesicular nanosystem formulations of traditional active ingredients for the treatment of acne – eg, benzoyl peroxide, tretinoin and adapalene – have demonstrated controlled active ingredient release and increased inhibition of acne-causing bacteria.
The increased surface area of the nanoparticles may contribute to the skin permeation into the stratum corneum and shows a better skin targeting effect with low or no systemic uptake of the active ingredient, when compared with the traditionally formulated reference products. It has been shown that lipid nanoparticles can form a thin film on the skin surface, which provides improved hydration and, as a result, penetration of the formulations through the skin.
Fungal nail infection

Figure 3: Representative image of nanoparticles.
Fungal nail infection, also known as onychomycosis, is a contagious infection of the nail plate and bed caused by dermatophyte fungi. It is one of the most common nail ailments, accounting for around 50 percent of all diseased nails.8 The ‘gold standard’ of drug treatments for onychomycosis is oral antifungal medicines, such as terbinafine hydrochloride, which are effective, but accompanied by significant safety concerns due to systemic exposure. Therefore, development of an efficacious topical treatment containing terbinafine hydrochloride would be desirable.
Our own research suggests that terbinafine hydrochloride, a highly hydrophobic drug, permeates through the nail plate at a much lower rate than hydrophilic drugs.9 By creating a new nanoparticle formulation of terbinafine with the amphiphilic polymer PHMB (polyhexanide), we aim to enhance topical nail penetration while maintaining the anti-fungal properties of terbinafine. We hypothesize that PHMB and terbinafine may form sub-structures that then aggregate into nanoparticles, and that the sub-structures may be of an appropriate size and hydrophilic character to penetrate the nail.
Conclusion
Nanotechnologies can help to tackle many of the challenges faced by the pharmaceutical industry when it comes to skin and nail drug delivery. Dermatological conditions are the ideal place to start when exploring the possibilities of nanomedicines, offering real potential to make a difference for many patients who still suffer from these often debilitating, chronic diseases.
About the authors


Dave Edwards joined Blueberry Therapeutics as Pharmaceutical Development Director in 2019 and has 12 years’ experience in the field of drug development. Dave holds a PhD in Chemistry from The University of Manchester and previously held the role of Development Manager at Teva Pharmaceuticals, specialising in project and technical management for drug product development. His areas of expertise include synthetic organic chemistry, molecular interactions, stability prediction, degradation mechanistics and pharmaceutical formulation.


A biochemist with nearly 20 years’ experience, including 17 years at AstraZeneca, Dave Cook joined the senior team at Blueberry Therapeutics in 2014 as Chief Scientific Officer (CSO). With a background in big pharma, Dave was keen to move into biotech as a way of exploring new research and development opportunities that would improve people’s lives. In his role at Blueberry Therapeutics, he is responsible for leading research activities for pre-clinical projects, with a focus on developing treatments for a range of dermatological disorders including fungal nail infection, acne and athlete’s foot.
References
- Vitorino C. Overcoming the skin permeation barrier: Challenges and Opportunities. Curr Pharm Des, 28, 2698 (2015).
- Walters KA. Dermatological and Transdermal Formulations (2002).
- Roskos KV. Percutaneous absorption in the aged. Dermatol Clun 4, 455 (1986).
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998; 11(3):415–429.
- Damiani G. 2019. Nanotechnology meets atopic dermatitis: Current solutions, challenges, and future prospects. Insights and implications from a systematic review of the literature. Bioactive Materials 4 (2019) 380-386.
- Kakkar V. 2019. An overview of atopic dermatitis with a focus on nano-interventions. EMJ Innov 2019; 3(1):44-54
- Paiva-Santos AC. 2021. Nanotechnology-based formulations towards the improved topical delivery of anti-acne active ingredients. Expert Opinion on Drug Delivery DOI: 10.1080/17425247.2021.1951218.
- Thomas J. 2010. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther 2010; 35(5): 497-519.
- Davies-Strickleton H. Assessment of the nail penetration of antifungal agents, with different physicochemical properties. Plos One (2020), 15(2): e0229414.
Issue
Related topics
Active Pharmaceutical Ingredient (API), Drug Delivery Systems, Drug Development, Drug Safety, Drug Targets, Formulation, Nano-medicine, Nanoparticles, Research & Development (R&D), Technology, Therapeutics
Related drugs
Related diseases & conditions
acne, Atopic Dermatitis, Eczema, fungal infections, Psoriasis, Skin cancer









