Laboratory Automation: Liquid handling devices in drug discovery – when, what, why?
Posted: 15 December 2013 |
Liquid handlers are ubiquitous and essential tools in every aspect of the drug discovery arena. Innovations in the past few decades resulted in a sizeable array of devices. With so many choices, it is important to identify appropriate instrumentation for a particular screening strategy, which should be based on unique capabilities and limitations.
Intense advances in the design of liquid handling devices have broadened the capabilities to screen larger collections of compounds at a faster pace with increased reliability and efficiency. These innovations drift towards miniaturisation, in large part to reduce cost and increase throughput. A wide selection of fluid handlers has been developed for every aspect of drug discovery, which incorporate different technologies for discrete functions. Although this segment focuses on instrumentation relevant to the screening of small organic molecules, the perspectives presented herein can be valuable in the handling of oligonucleotides or biologics.
Liquid handlers can be broadly categorised as bulk dispensers or transfer devices, depending on whether large volumes of a single reagent are being distributed or smaller quantities are being transferred1. In addition, these instruments can follow two dispensing modes: contact or non-contact, depending on whether the fluid to be transferred is allowed to touch the surface of the destination container or solution2. Based on the delivery mechanism, they include tip-, piezoelectric-, solenoid- and acoustic-based devices in addition to peristaltic pumps and pin-transfer3:
- The peristaltic pump is used for bulk reagent dispensing, where a continuous fluid motion is created in tubing compressed by rotating rollers
- Tip-based transfer devices can incorporate fixed or changeable (disposable) tips assembled in 2-, 4- or 8-channel expandable liquid handling arms in addition to 96- or 384-channel heads. They function based on an air displacement mechanism; in some cases the dilutor or syringe plunger pulls system liquid from the pipette tubing to aspirate the sample, with an air gap separating both fluids. In other situations, only air is displaced to drive liquid movement
- A pintool device consists of a cassette with an array of floating stainless steel pins. The end of the pins that is in contact with the reagent can follow various patterns, including solid, grooved or slotted surfaces. An optional hydrophobic coating is intended to reduce non-specific binding. Solutions are transferred through a combination of capillary action and surface tension, with the volume being highly dependent on contact surfaces and solution properties
- The piezoelectric dispenser delivers solutions as multiple tiny drops of defined size. A piezoelectric crystal collar is bound to a capillary made of quartz or steel, which is filled with the desired reagent. After applying voltage, the piezoelectric element contracts ejecting fine drops through an orifice at the end of the capillary
- Solenoid-based devices make use of solenoid valves to occlude the flow of pressurised liquid. Electric current actuates the valves, allowing the solution to flow as droplets or continuous stream
- Acoustic droplet ejection (ADE) adopts acoustic energy to propel droplets from a source plate to any well in the destination plate, which remains in an inverted position. Specialised lab ware is required for this technology.
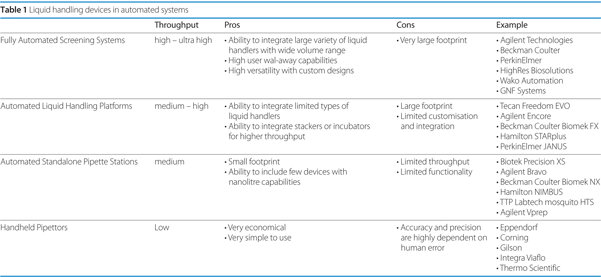
Many of the various kinds of liquid handlers can be utilised as a standalone component, functioning independently from any other equipment. However, these devices are normally integrated into larger stations that facilitate routine tasks, reduce human intervention, increase throughput and allow for multi-tasking in a single run (Table 1). These systems can range from compact workstations dedicated for a particular purpose, to fully-automated robotic decks capable of performing an innumerable combination of tasks.

Broadly speaking, one can consider two major kinds of solutions or suspensions that partake in a typical screening endeavour: biological reagents and compounds to be tested. They may require devices with differing mechanisms, as the latter is normally delivered at small quantities, while the biological mixtures are typically dispensed in bulk.
Transfer of biological reagents
Cells, proteins, oligonucleotides or other biological components that comprise the assay are allocated uniformly throughout the assay plate in a column- or row-wise fashion, for which bulk-reagent dispensers are used. Peristaltic pumps or pressurised-dispensers are commonly employed, which are capable of delivering variable volumes from submicrolitre to microlitre range with high speed and consistency. Although they are widely integrated in robotic platforms for medium to higher throughput systems, they can readily be used as a standalone module or in conjunction with stackers for experiments with lower output.
When minimum reagent waste is desired (minimal dead-volume), or dealing with viscous solutions, automated pipettors equipped with 8-, 12-, 96- or even 384-tips can be employed. They are generally devoted to low throughput projects due to the inherent lower speed or higher costs associated with consumables.
Transfer of compounds
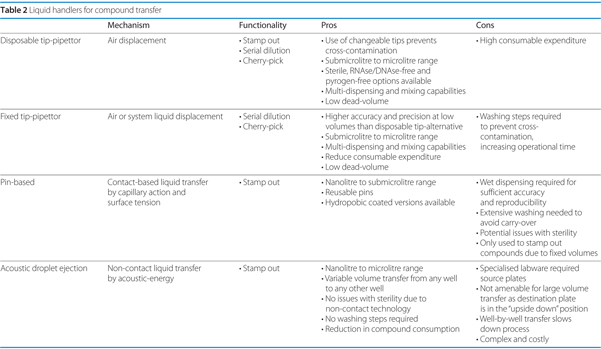
The chemical library is the most valuable asset in any screening facility, which comprises commercially available and in-house synthesised compounds. The success of a campaign depends heavily on the proper manipulation and preparation of these entities: some of the key aspects to consider include purity preservation by reducing cross-contamination and ensuring accurate concentrations. A considerable array of tools is available at disposal to perform the various applications associated with compound manipulation (Table 2):
- Stamp-out: simultaneous transfer of the contents of an entire source plate for the generation of replica copies, compression to higher density formats or plate-to-plate dilution
- Cherry-pick: selection and transfer of particular compounds from the chemical repository for confirmatory, retest and further analysis
- Serial-dilution: stepwise dilution of substances over a wide concentration range with the aim to generate dose- or concentration-dependent curves.
Regardless of the size of a screening centre, the first major phase encompasses compound reformatting, wherein chemicals are condensed from lower to higher density microtitre plates, and copies are generated for screening, long-term storage or cherry-pick4. Traditionally, this stage is most efficiently accomplished by stamping-out the entire source plate to the destination plate using pipettors typically with 96-, 384- or even 1536-channel heads in automated standalone or fully-integrated systems, but acoustic-energy based devices have been progressively introduced to fulfil this important task.

At the heart of a screening process, compounds are mixed with biological materials, which are transferred during the run from a stock plate that can be used repeatedly, or dispensed beforehand to form assay-ready plates for single use. As illustrated in the following segment, the various approaches in the delivery of compounds highlight the complexity in designing the appropriate protocols and selecting suitable liquid transfer equipment.
At the High Throughput Screening Center at St. Jude Children’s Research Hospital, pintool is used to transfer small volumes of concentrated compound solutions (in DMSO) from source plates to assay plates prefilled with biological material. This strategy requires that all the compounds in the repository to be properly formatted in suitable 384-well polypropylene plates and readily available for screening at single-concentration. Subsequent experiments in a dose-response manner of a more limited set of compounds involve cherry-pick from individual tubes followed by dilution using fixed 8-channel pipette systems. These compound plates can be used repeatedly, as only a fraction is being transferred by pintool. Space requirement for plate storage becomes an issue with increasing numbers of projects. We maintain our copies in a refrigerated storage facility with humidity control and an integrated robot for plate and tube retrieval.
Some other major facilities pursue a drastically different strategy, relying mainly on the versatility of acoustic-based devices, which allow for screening of specific subset of compounds at single or multiple concentrations. The choosing at will of particular chemicals provides great flexibility when different compounds are needed for various projects, and the versatility to transfer varying amounts eliminates the need for additional instrumentation that otherwise would be needed to conduct cherry-pick and dilution.
The use of assay-ready plates is a strategy that is commonly employed in many laboratories with limited budget and resources, since the process is simplified and can be easily implemented using standalone equipment, although the approach is also suitable for integrated robotic decks. This permits for a drastic reduction of screening or deck time by bypassing ‘drugging’ or the transfer of compounds between plates, as transfer devices such as pintool are oftentimes the bottleneck in a screening cycle. Although plates containing pre-dispense compound solutions can be prepared by a variety of liquid handlers, including the acoustic-based devices, small centres lean towards using economical automated pipettor systems with fixed or disposable tips. Because pipettors handle relatively large volumes (microliter range), extra steps are needed to reduce the DMSO content by using intermediate plates, which have lower DMSO levels than the stock plates and yet unacceptably high organic-solvent amount for final assay conditions. Regardless of whether the preparation of assay-ready plates is performed by automated pipettors or acoustic-based handlers, the process is relatively slow and needs to be done in advance. The downside to this approach is that long-term storage of chemicals in highly aqueous solutions may lead to compound precipitation and degradation. Moreover, these plates are for single-use only, and multiple copies are required to profile the same set of compounds against multiple targets. The assay type of future experiments needs to be anticipated, as different plate materials are intended for distinct read-outs: solid back / white and clear-bottom plates for fluorescence / luminescence and absorbance, respectively.
The careful selection of liquid handling devices can have detrimental effects down the road in terms of cost: equipment procurement and maintenance, consumable expenditure and reagent waste. For a small facility, it is sufficient to employ compact or medium-size workstations with multi-functional capabilities, competent in executing all major tasks (Table 1). As operations expand, a higher degree of specialised machines are needed to fulfil a growing number of divergent projects. Nonetheless, it is noteworthy to point out that processes that deal with large number of plates do not necessarily demand an extensive collection of fluid handlers. For instance, quantitative HTS (qHTS) can be undertaken by inter-plate based dilutions instead of the traditional intra-plate alternative5, for which only a bulk dispenser, multi-channel pipettor for stamp-out, and pintool are required, thus eliminating the need for dedicated instruments for cherry-pick and serial-dilution.
The overall strategic vision for discovering therapeutically-relevant compounds dictates the appropriate instrumentation needed. Considerations in the selection of liquid handlers should include reliability, reproducibility, robustness to handle extensive experiments, ease in operation and maintenance, integration capabilities and cost. Emerging technologies that are capable of multitasking with a broad volume range can have a profound and positive impact on the drug discovery landscape.
Acknowledgment
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital, and National Cancer Institute grant P30CA027165.
References
- Rudnicki S, Johnston S. Overview of Liquid Handling Instrumentation for High-Throughput Applications. Current Protocols in Chemical Biology. 2009;1(1):43-54
- Kong F, Yuan L, Zheng YF, Chen W. Automatic liquid handling for life science: a critical review of the current state of the art. Journal of laboratory automation. 2012;17(3):169-85. Epub 2012/02/24
- Chai SC, Goktug AN, Cui J, Low J, Chen T. Practical Considerations of Liquid Handling Devices in Drug Discovery. 2013
- Cui J, Chai SC, Shelat AA, Guy RK, Chen T. An automated approach to efficiently reformat a large collection of compounds. Current chemical genomics. 2011;5:42
- Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(31):11473-8. Epub 2006/07/26
Biographies






Issue
Related topics
Drug Discovery, HTS (High Throughput Screening), Lab Automation









