Finding novel targets for anticancer target discovery
9 May 2010 | By Wolfgang Link, Experimental Therapeutics Program, Centro Nacional de Investigaciones Oncologicas (CNIO)
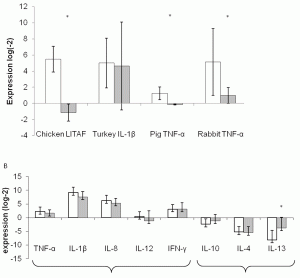
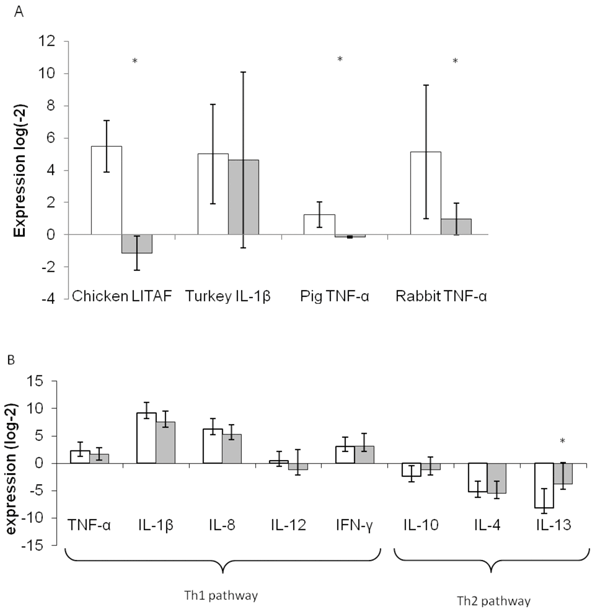
Advances in our understanding of the molecular basis of cancer together with novel approaches to interfere with signal transduction pathways have opened new horizons for anticancer target discovery. In particular, the image based large scale analysis of cellular phenotypes that arise from genetic or chemical perturbations paved the way for…