Applying systems biology and computer simulations to predicting idiosyncratic DILI
19 August 2010 | By David Cook, Associate Director, Global Safety Assessment, AstraZeneca
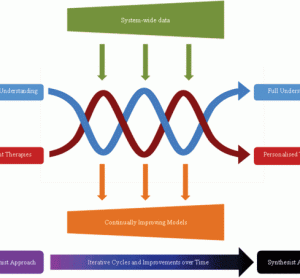
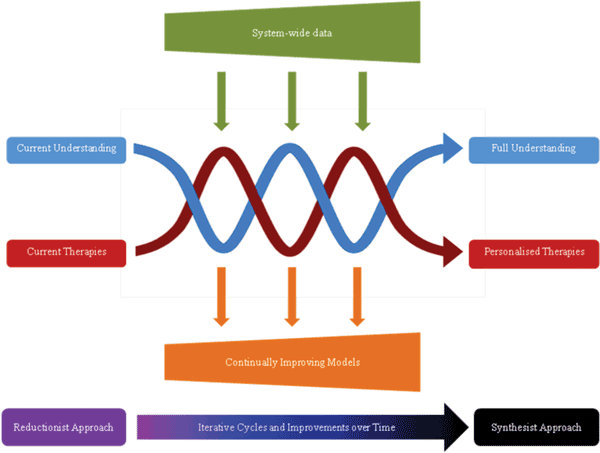
Idiosyncratic drug-induced liver injury (DILI) is a rare adverse drug reaction which accounts for a significant amount of patient suffering, including death. Currently, idiosyncratic DILI is unpredictable and as a result arises late in the drug development process or even post-marketing. The prediction of idiosyncratic DILI based on preclinical or…