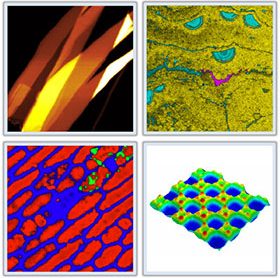
HORIBA Scientific launches Swift v.2 for ultra fast, high definition confocal raman imaging
20 March 2015 | By HORIBA
HORIBA Scientific, global leader in Raman and optical spectroscopy systems for over 50 years, announces the release of their newest SWIFT v2 ultra fast confocal Raman imaging module...