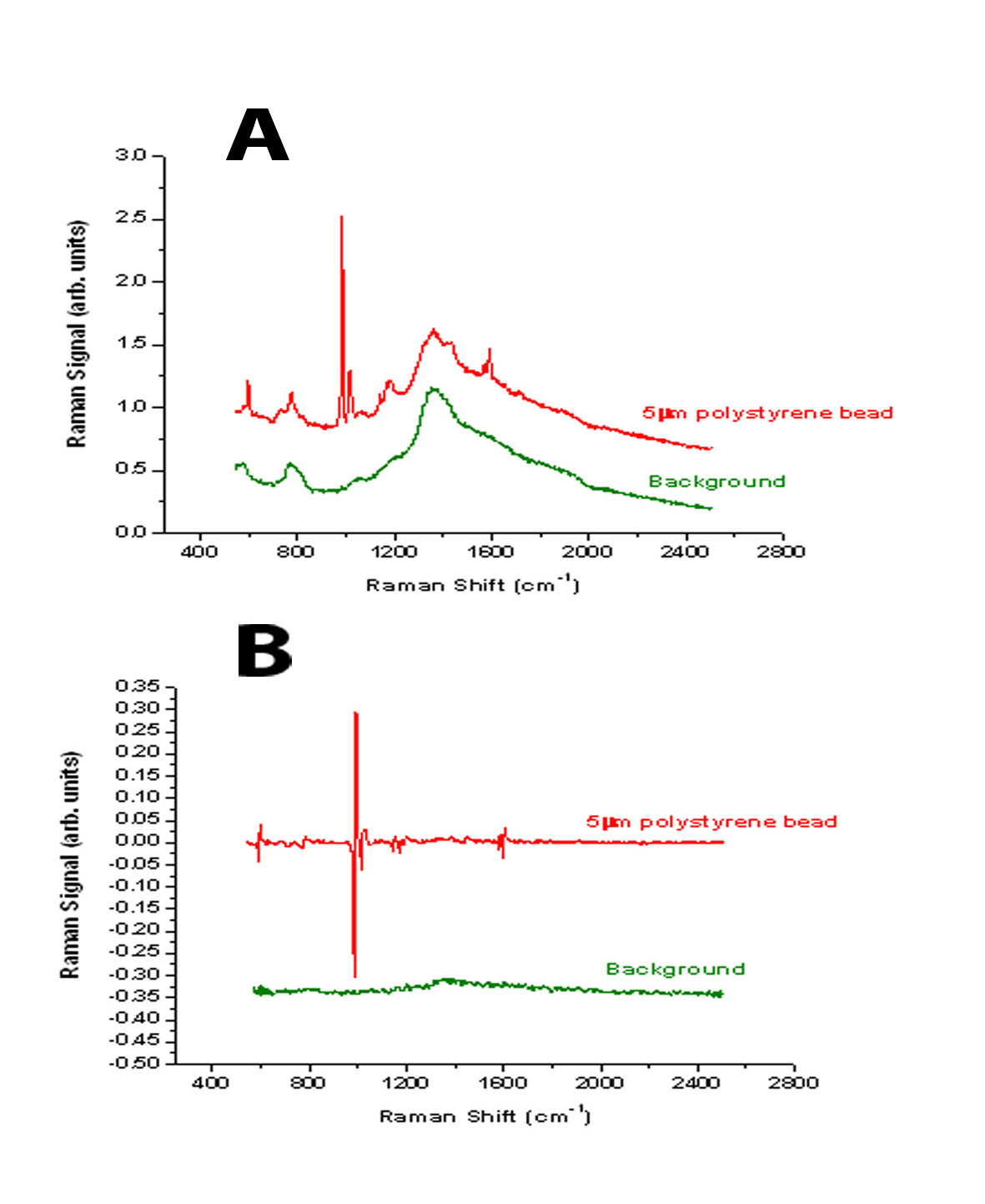
Primary packaging materials for pharmaceutical freeze-drying: Moulded vs. serum tubing vials
19 August 2010 | By Susanne Hibler and Dr. Henning Gieseler, University of Erlangen-Nuremberg, Division ofPharmaceutics, Freeze Drying Focus Group
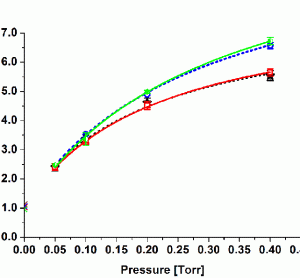
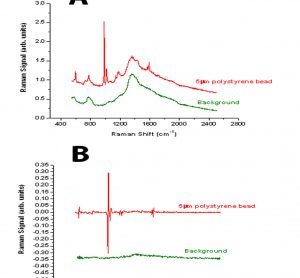
Pharmaceutical freeze-drying is used to stabilise delicate drugs which are typically unstable in solution over a longer shelf life. The liquid formulation is converted into a solid, highly porous cake which can be easily reconstituted prior to administration. The majority of freeze-dried products in the pharmaceutical industry are used for…