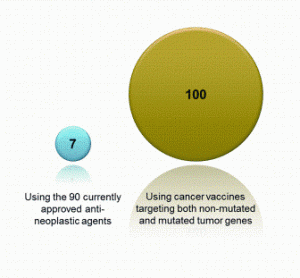
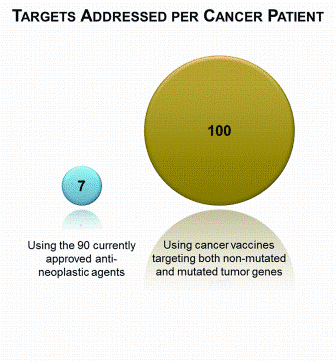
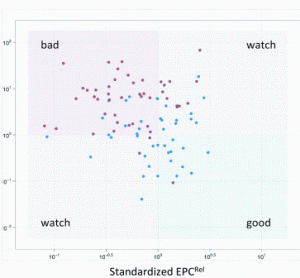
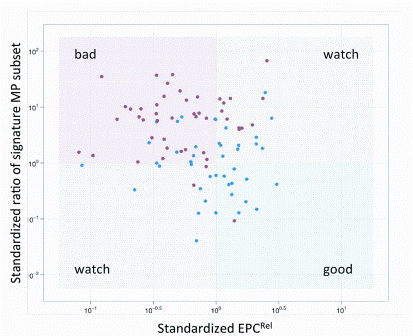
Opportunities for rapid methods discussions: where the experts are meeting!
3 September 2012 | By Michael J. Miller, President, Microbiology Consultants, LLC
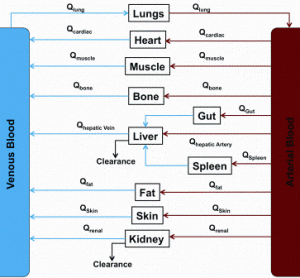
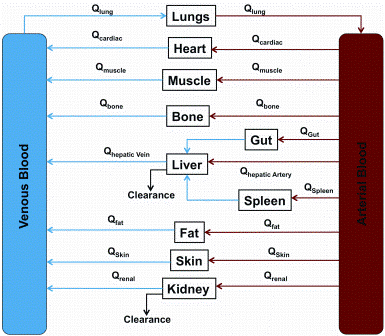
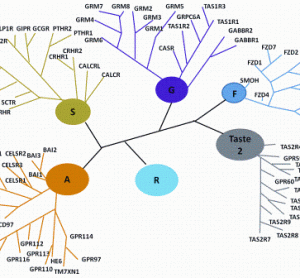
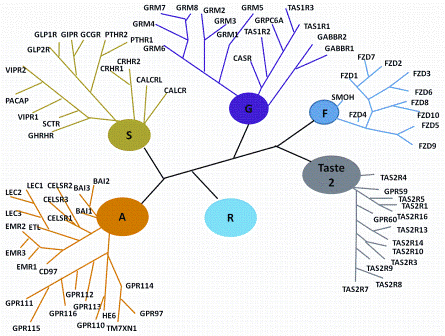
This is the fourth paper in our continuing series on Rapid Microbiological Methods (RMM) that will appear in European Pharmaceutical Review during 2012. Over the past few years, a number of professional meetings have focused on strategies and case studies for the validation and application of rapid microbiological methods (RMM).…