Novel antibody delivery method could improve precision medicine
Posted: 23 April 2020 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
Scientists have developed a protocol for encapsulating antibodies in an ultrasound-sensitive drug carrier for targeted drug delivery.
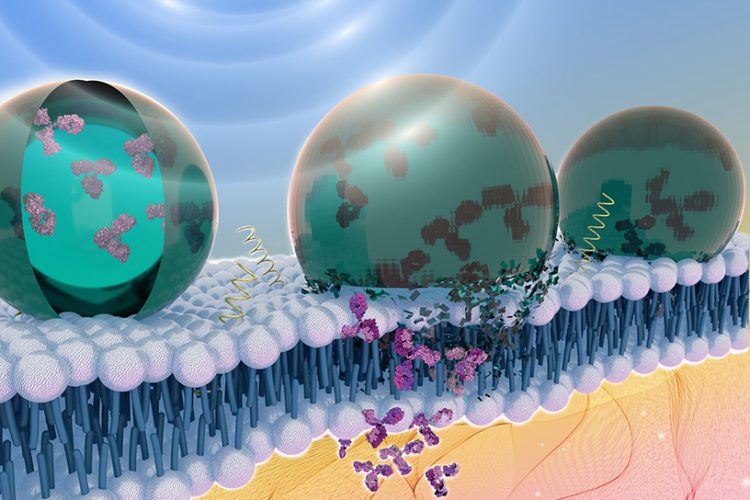

A team of Penn State researchers are interfacing chemical biology and nanotechnology to develop ultrasound-controlled nanomaterials that can provide on-demand, high precision delivery of proteins into human cells [credit: Jennifer McCann/Penn State].
Researchers have developed a novel method for delivering therapeutic proteins to precise targets within the body. Their solution was to encapsulate the proteins in acoustically sensitive carriers which, in response to ultrasound, release their payload to a target.
“When you expose the particle to ultrasound it opens a hole in the cell membrane that lasts for a couple of microseconds,” said Scott Medina, assistant professor of biomedical engineering at Penn State. “We can use this temporary opening to deliver antibodies, which are attractive therapeutic molecules in precision medicine that cannot otherwise get inside cells.” These antibodies could be emerging therapeutics for cancers, infectious disease and rheumatoid arthritis.
However, the team revealed that successfully encapsulating the antibodies was difficult, because the protein did not interact well with the fluorous liquid that makes up the interior of the particle. In order to combat this, Janna Sloand, Medina’s doctoral student and first author of the paper published in ACS Nano, suggested using a chemical mask to coat the protein and allowing it to interact with the fluorous liquid. They found the fluorous mask had the correct counterbalance or polarity and fluorine to maintain the folded state and bioactivity of the protein, while enabling it to interact with the fluorous liquid.
“We had a lot of challenges developing this new method,” said Sloand. “The most difficult was figuring out what kind of chemicals could mask the protein. That was definitely my eureka moment when I saw that it worked.”
The team now plan to explore how their ultrasound-programmable material could be used as a platform for image-guided delivery of therapeutic proteins and gene editing tools. They are already using the technology to deliver antibodies that can alter abnormal signalling pathways in tumour cells, turning off their malignant traits and delivering gene editing tools, like CRISPR constructs, to enable ultrasound-controlled genome engineering of cells in complex three-dimensional (3D) tissue microenvironments.
They highlighted that a key feature of their technology is that the ultrasound required is the same as that already employed for other applications in hospitals, potentially making implementation of their technology easier in future.
Related topics
Anti-Cancer Therapeutics, Drug Delivery Systems, Gene therapy, Nanoparticles, Proteins, Respiratory Drug Delivery (RDD), Therapeutics









