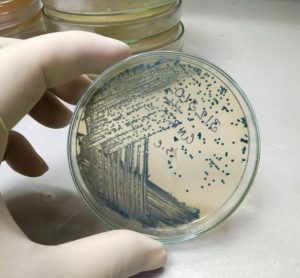
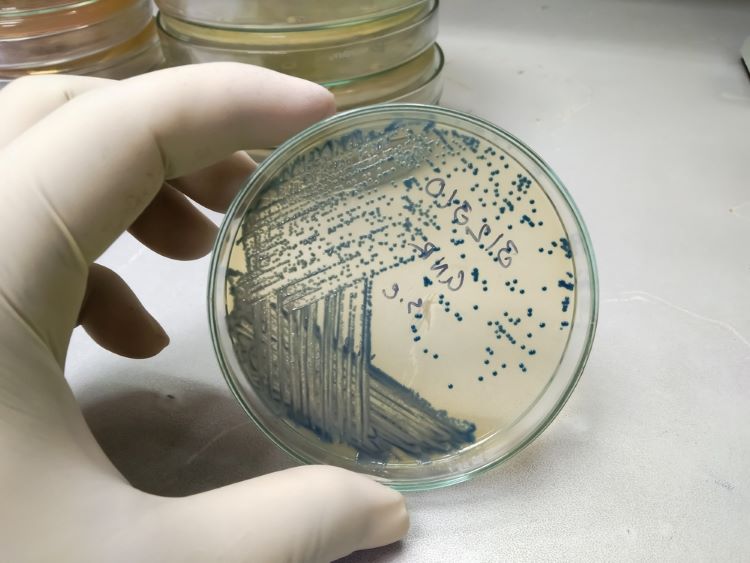
Validation data for the new MAS-100 Sirius
In this App Note, MBV AG showcases the MAS-100 Sirius as the advanced successor to the MAS-100 NT, offering significantly enhanced capabilities for quantitative monitoring of airborne viable particles in cleanrooms.