Physicochemical failure modes for first-line therapy Narrow Therapeutic Index (NTI) drugs: a call for attention NTI risk classification and New Prior Knowledge
Posted: 2 July 2021 | Dr Ajaz Hussain, Dr Harsh S. Shah (J-Star Research Inc), Dr Kaushalendra Chaturvedi (J-Star Research Inc), Dr Kenneth Morris (Long Island University) | No comments yet
In this article, Dr Harsh Shah and colleagues summarise their research into the importance of characterising physicochemical characteristics that can result in stress-induced solid-state changes in Narrow Therapeutic Index (NTI) drugs.


Abstract
Historically, categorisation of Narrow Therapeutic Index (NTI) drugs has primarily focused on a therapeutic index – a quantitative safety measure calculated by comparing of the median lethal dose to the median therapeutic dose of a drug. Drugs with narrow therapeutic windows (ie, a small range of dosages which can treat disease effectively without having toxic effects) demand precise control of stability and bioavailability because small variations in strength and/or delivery can directly impact safety and efficacy, resulting in clinical failures. NTI drug substances prone to stress-induced solid-state changes can be at the root of clinical failures and not acknowledged because they are often not characterised. Here, Dr Harsh Shah, Dr Kaushalendra Chaturvedi, Dr Ajaz Hussain and Dr Kenneth Morris summarise their research which highlights this gap with three NTI drugs – levothyroxine, warfarin and phenytoin – to call attention to the need to improve the classification of NTI drugs by considering physicochemical failure modes. They also propose a border solution conceptualised as “New Prior Knowledge” to enhance the efficacy of generic drug development and the US Food and Drug Administration (FDA) review process.
Introduction
Levothyroxine sodium (hypothyroidism treatment), warfarin sodium (anti-coagulant) and phenytoin sodium (anti-epileptic agent) are first-line therapy NTI drugs. Each is prescribed to a large segment of the population; for instance, in the US in 2018, levothyroxine sodium was prescribed over 105 million times, warfarin sodium almost 15 million times and phenytoin sodium over 1.6 million times.1 As a result, failure of products to meet specification and stability failures result in recurring recalls. The situation is also complicated as the assurance of therapeutic equivalence is variable.


Hypothyroidism is the inability of someone to produce thyroid hormones. This deficiency affects patients across the spectrum of the American population; however, is more consequential in African Americans, American Indians/Alaska Natives and Pacific Islanders. Despite the availability of levothyroxine sodium for over half a century, these products still suffer from recurring, unresolved quality and safety issues, as evidenced in an April 2021) recall of 35 lots of Acella Pharmaceuticals’ NP Thyroid® tablets (containing levothyroxine and liothyronine).2 During that recall Acella received 43 reports of serious adverse events.3 There are approximately 26.4 million patients who take levothyroxine sodium and almost four million patients still suffer from refractory hypothyroidism – a condition in which the physicians continue prescribing the next higher dose of levothyroxine sodium to gauge the effective hypothyroidism treatment in the patients. Shah et al. suggest monitoring therapeutic drug concentrations is also impacted by quality compliance issues during manufacturing and storage.
Solid-state instability and physicochemical properties of drug substances
In their work Shah et al. speak to fundamental aspects of solid-state instability linking to physicochemical properties of drug substances during the manufacture of drug products and storage that then lead to chemical degradation. For instance, dehydration of the levothyroxine sodium pentahydrate precedes chemical degradation.4 Dehydration of levothyroxine sodium pentahydrate can occur under stress conditions in manufacturing, such as high temperatures, low humidity conditions and mechanically induced stress.5 In their studies, Shah et al. found this degradation can lead to a sub-potent drug product.4, 6, 7 For example, a prescription of 100µg dose will deliver only 80µg, with 20 percent chemical degradation – which is closer to the next lower marketed dose, potentially decreasing the therapeutic effect contributing to continuing hypothyroidism.


Levothyroxine sodium has been available as a pentahydrate8 for drug product manufacturing. Similarly, warfarin sodium as 2-propanol solvate9 and phenytoin sodium in its anhydrate form.10 Shah et al.’s research points to several different solid forms for each of these NTI drug substances and uses advancements in computational materials science to predict the physicochemical properties of a drug substance using a single-crystal structure.
Their research elucidates single-crystal structures of anhydrate levothyroxine sodium, anhydrate warfarin sodium and phenytoin sodium mixed methanol hydrate.4, 11, 12 In addition, it integrates predictive modelling, statistical analysis and qualitative analysis to characterise the solid form of drugs in the final products. Beyond new drug development, their work offers tools to evaluate marketed products and improve investigations of quality failures.
For instance, a case study on the anti-coagulant drug warfarin sodium demonstrates that this drug, a crystalline 2-propanol solvate, can undergo solid-state changes under high humidity and/or elevated temperatures to form a desolvated form and a non-crystalline form. These solid forms have significantly different solid-state properties11, 13 and can lead to variability in drug solubility and incomplete dissolution due to disproportionation of the salt form. The sodium salt with a very high solubility de-protonates to a unionised free acid with a limited aqueous solubility raising questions and concerns about variable bioavailability and monitoring of warfarin treatment.14 The maintenance of appropriate in vivo warfarin levels is critical, as variances can be consequential in healthcare practice and may result in fatalities. A third case study is that of phenytoin sodium. This anti-epilepsy drug can form a hydrate at high humidity conditions and is very hygroscopic (ie, it tends to absorb moisture from the air).12 The hydrate form of phenytoin sodium had a limited solubility. Like warfarin sodium, phenytoin sodium also disproportionates to phenytoin free acid leading to decreased dissolution.15
New Prior Knowledge – a border solution
Characterisation of solid-state physicochemical characteristics discussed here ideally occurs during the pre-formulation phase to inform and guide the development of stable and bioavailable products. These attributes are curated in public drug monographs to be part of prior knowledge for effective generics drug development and FDA assessment of generic applications identifying failure modes (areas of potential product failure), assessing risks and ensuring quality by design. Attention on all relevant failure modes will aid in more reliable delivery of successful therapies to treat or cure incurable diseases such as autoimmune diseases, cancer diseases (eg, lung cancer, mesothelioma and neuroblastoma), cardiovascular diseases, lung disorders, cystic fibrosis and hormonal deficiency disorders.
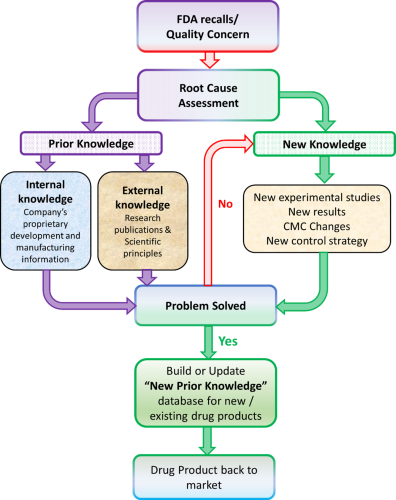

Figure 1: Decision tree for managing drug product recalls with the help of “New Prior Knowledge.”
However, the current system for physicochemical corrections in the context of pre-formulation, formulation development and knowledge curation continues to be neglected even for NTI drugs. This research also serves as an indicator for the urgent need to improve the classification of NTI drugs considering physicochemical failure modes and proposes a border solution conceptualised as “New Prior Knowledge” to enhance the efficacy of generic drug development and FDA review process.16
Characterising solid forms at the molecular level is foundational to a chemistry, manufacturing and control classification system for NTI drugs. Such a classification system can evolve from a process, conceptualised in Figure 1, for investigating quality failures to correct and prevent reoccurring failures and generate New Prior Knowledge. Publicly available New Prior Knowledge will facilitate efficient development of generic drugs, reduce product failures and recalls and improve assurance of therapeutic equivalence for NTI drug products. A pharmaceutical product development report (eg, ICH CTD P2 Section) identifying solid-state physicochemical failure modes and a control strategy to mitigate the risk of failure will improve product risk assessment, ensure quality by design, reduce batch rejection, product recalls and give patients, the public and practitioners the assurance of therapeutic equivalence they need for NTI drugs products.
Overall, this award winning doctoral dissertation is an important source of new knowledge and novel solutions, correcting assumptions and contributing to New Prior Knowledge.17 It highlights the current gaps in the characterisation process for NTI drugs, as well as posing a potential solution for the problem in the concept of New Prior Knowledge.
About the authors


Dr Harsh S. Shah has more than 10 years of experience in pharmaceutical field with his expertise in identifying solid forms with improved physico-chemical properties, drug product risk assessment and formulation studies. He is currently working as a senior scientist at J-Star Research Inc. in the US where he leads “fit-to-purpose” projects in drug discovery and drug product design. His prior experience includes the Lachman Institute for Pharmaceutical analysis at Long Island University, collaborative projects with the United States Food & Drug Administration (FDA), Bristol Myers Squibb, Triclinic Labs, Vertex Pharmaceuticals and Amneal Pharmaceuticals.


Dr Kaushalendra Chaturvedi is currently working as a Senior Research Scientist at J-Star Research. He is an experienced pharmaceutical scientist with the skills supporting pre-formulation and materials science activities. Dr Chaturvedi received his PhD in Pharmaceutics and Drug Design from the Long Island University (LIU) in 2019 and MS in Pharmaceutical Sciences and Bachelor in Pharmacy from Pune University in 2011 and 2009 respectively. During his doctoral study he worked on multiple FDA, pharmaceutical and excipient industry sponsored projects.


Dr Ajaz Hussain’s career spans academia, FDA, industry and private practice. A Fellow of AAPS and the Swiss Society of Pharmaceutical Science, he currently explores life-in-science in his writings and advising companies. Previously, the President of the National Institute for Pharmaceutical Technology and Education, Deputy Director in the Office of Pharmaceutical Science at CDER, VP and Global Head Biopharmaceutical Development at Sandoz, Chief Scientific Officer at Philip Morris International Scientific Officer and President Biotechnology at Wockhardt. His career started in academia after completing his doctoral degree from the University of Cincinnati. He trained as a pharmacist in Mumbai.


Dr Kenneth Morris has worked in the field of pharmaceutical materials science for more than 30 years. This area can be described as the study of the impact of the physico-chemical properties of formulation components on the performance of the final pharmaceutical dosage form with a focus on the use of advanced analytical techniques to follow these properties throughout the manufacturing process. He was a chemist at the US EPA, a research scientist at BMS, then a Professor and associate department head in the IPPH department at Purdue University. He is a retired University Professor and Director of the Lachman Institute for Pharmaceutical Analysis at Long Island University, a Professor emeritus at the University of Hawaii at Hilo, a special government employee of the US FDA-CDER-OPQ and an adjunct Professor at Purdue University.
References
- ClinCalc DrugStats Database. https://clincalc.com/DrugStats/ [Accessed 21 May 2021]
- United States Food and Drug Administration, Acella Pharmaceuticals, LLC, Issues Voluntary Nationwide Recall of Certain Lots of NP Thyroid® (Thyroid Tablets, USP) Due to Sub Potency. https://www.fda.gov/safety/recalls-market-withdrawals… [Accessed 21 May 2021]
- Acella Pharmaceuticals Issues Voluntary Nationwide Recall of Certain Lots of NP Thyroid®. https://www.americanpharmaceuticalreview.com/… [Accessed 21 May 2021]
- Shah HS, Chaturvedi K, Hamad M, Bates S, et al. New Insights on Solid-State Changes in the Levothyroxine Sodium Pentahydrate during Dehydration and its Relationship to Chemical Instability. AAPS PharmSciTech 2019, 20, (1), 39.
- Collier JW, Shah RB, Gupta A, Sayeed V, et al. Influence of Formulation and Processing Factors on Stability of Levothyroxine Sodium pentahydrate. Aaps Pharmscitech 2010, 11, (2), 818-825.
- Kaur N, Young Jr VG, Su Y, Suryanarayanan R. Partial Dehydration of Levothyroxine Sodium Pentahydrate in a Drug Product Environment: Structural Insights into Stability. Molecular pharmaceutics 2020, 17, (10), 3915-3929.
- Patel H, Stalcup A, Dansereau R, Sakr A. The Effect of Excipients on the Stability of Levothyroxine Sodium Pentahydrate tablets. International journal of pharmaceutics 2003, 264, (1-2), 35-43.
- Katrusiak A, Katrusiak A. Thyroxine Revisited. Journal of pharmaceutical sciences 2004, 93, (12), 3066-3075.
- Sheth AR, Young Jr VG, Grant DJ. Warfarin Sodium 2-propanol Solvate. Acta Crystallographica Section E: Structure Reports Online 2002, 58, (5), m197-m199.
- Camerman A, Camerman N. The Stereochemical Basis of Anticonvulsant Drug Action. I. The Crystal and Molecular Structure of Diphenylhydantoin, a Noncentrosymmetric Structure Solved by Centric Symbolic Addition. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 1971, 27, (11), 2205-2211.
- Shah HS, Chaturvedi K, Dave RH, et al. New Insights on Warfarin Sodium 2-Propanol Solvate Solid-State Changes Using a Multivariate Approach. Crystal Growth & Design 2020, 20, (11), 7328-7340.
- Shah HS, Chaturvedi K, Zeller M, et al. A Threefold Superstructure of the Anti-epileptic Drug Phenytoin Sodium as a Mixed Methanol Solvate Hydrate. Acta Crystallographica Section C: Structural Chemistry 2019, 75, (9), 1213-1219.
- Sheth AR, Brennessel WW, Young VG, et al. Solid‐state Properties of Warfarin Sodium 2‐propanol Solvate. Journal of pharmaceutical sciences 2004, 93, (11), 2669-2680.
- Shah HS, Chaturvedi K, Dave RH, Morris KR. Molecular Insights into Warfarin Sodium 2-Propanol Solvate Solid Form Changes and Disproportionation Using a Low Volume Two-Stage Dissolution Approach. Molecular Pharmaceutics 2021, 18, (4), 1779-1791.
- Rahman Z, Dharani S, Ali SFB, Afrooz H, et al. Effect of Processing Parameters and Controlled Environment Storage on the Disproportionation and Dissolution of Extended-release Capsule of Phenytoin Sodium. International journal of pharmaceutics 2018, 550, (1-2), 290-299.
- Hussain AS, Gurvich VJ, Morris K. Pharmaceutical “New Prior Knowledge”: Twenty-First Century Assurance of Therapeutic Equivalence. AAPS PharmSciTech 2019, 20, (3), 140.
- Shah HS. Understanding and Classifying the Solid-State Properties of Selected Narrow Therapeutic Index Drug Substances and Modeling the Contribution of Stress Induced Changes on Drug Product Failure Modes. Long Island University, The Brooklyn Center, 2019.
Related topics
Related organisations
Acella Pharmaceuticals, US Food and Drug Administration (FDA)









