UK at the forefront of advanced therapies
Posted: 26 August 2021 | Ceri Roberts (NHS Blood and Transplant), Rachel Bell (Marks & Clerk) | No comments yet
Advanced therapies are a ground-breaking new class of medicines that use gene therapy, cell therapy or tissue engineering to treat disease and injury. Rachel Bell, Trainee Patent Attorney at Marks & Clerk, and Ceri Roberts, Scientific Training Manager – Cellular and Molecular Therapies at NHS Blood and Transplant, discuss some important developments in the field and how the UK is playing a central role in realising their potential.
![Researcher looking at an experiment [Credit: Unsplash].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Bell-advanced-therapies-feature.jpg)
![Researcher looking at an experiment [Credit: Unsplash].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Bell-advanced-therapies-feature.jpg)
[Credit: Unsplash].
ADVANCED THERAPIES are novel treatments that offer exciting therapeutic options for a wide range of indications – with the potential to be long lasting or even curative – and can address complex and rare disorders for which there are no effective treatments.
The UK is at the forefront of this rapidly moving field, boasting nearly a quarter of Europe’s advanced therapy medicinal product (ATMP) developers’ headquarters and over 70 active companies.1 Additionally, the number of ATMP clinical trials in the UK is increasing each year: 154 trials were ongoing in the UK in December 2020, representing 12 percent of all global activity.2 Advanced therapies can be very expensive and challenging to develop, bring to market and, following regulatory approval, deliver to patients. These medicinal products come with particularly complex logistics and supply chain considerations, necessitating new ways of working and close communication between multiple teams across the commercial, academic and healthcare sectors.
Clearly, given the R&D activity surrounding advanced therapies, there is a growing market for investment, particularly in the UK. One recent success was the UK Government Industrial Strategy Challenge Fund awarding a five-year grant funding agreement between Cell & Gene Therapy Catapult, UK Research and Innovation (UKRI) and Innovate UK in 2018. This provided investment of £70.6 million for cell and gene therapy projects.3 With such innovation comes a huge amount of intellectual property (IP) which further drives investment in this area. The patent field of advanced therapies is growing, with increased filing numbers every year (according to data from PatSnap4), and the UK and Europe are showing stronger activity in this area.
The UK National Health Service (NHS) is leading innovation in this evolving industry, taking a proactive approach to addressing the practical challenges of implementation and working within alternative funding frameworks through programmes such as the UK Cancer Drugs Fund and the Accelerated Access Collaborative. To date, only a few ATMPs have been recommended by the National Institute for Health and Care Excellence (NICE) and made available to NHS patients, mostly on a small scale and often in a research context; but this is expected to change as the field continues its rapid growth.
The Advanced Therapy Treatment Centre (ATTC) network5 is a world-first programme operating within the NHS framework and co-ordinated across the UK by the Cell and Gene Therapy Catapult. Established in 2018 through funding from UKRI’s Industrial Strategy Challenge Fund, the ATTCs bring together industry, NHS and academic organisations to accelerate patient access to ATMPs by addressing the unique challenges of delivering these types of treatments. One such centre, the Midlands and Wales ATTC (MW-ATTC), comprises a consortium of healthcare, university and commercial partners with expertise in advanced therapy manufacturing, ATMP logistics companies, specialists in clinical trial delivery and teams focused on developing novel IT solutions and health economics analysis.
The UK is at the forefront of this rapidly moving field, boasting nearly a quarter of Europe’s advanced therapy medicinal product (ATMP) developers’ headquarters and over 70 active companies”
Several exemplar clinical trials are testing the innovative systems being developed within the MW‑ATTC programme, which has brought about extraordinary cross-sector engagement and collaboration – and generated tangible outputs within a short time frame. For example, Cytiva6 (Formerly GE Healthcare Life Sciences) has partnered with Orbsen Therapeutics,7 a biotechnology company developing a purified stromal cell immunotherapy (ORBCELTM), to conduct a pilot study of its Chronicle software to track ‘chain of custody’ of the cryopreserved product as it moves to clinical trial and storage sites across the UK.8 This kind of multi-sector partnership has allowed the constructive and proactive identification of potential barriers to delivering ATMPs, allowing solutions to be put in place and validated ahead of an anticipated increase in demand. The ATTC network has recently been awarded a further £9.5 million to fund an additional 12 months of the programme, supporting additional initiatives and continuation of projects that have been impacted by the COVID-19 pandemic.9
ATMPs require complex specialist manufacturing and logistics services, which may prove to be a limiting factor for cell and gene therapy developers. The UK’s national blood services have significant expertise in providing and distributing blood products, stem cells and organs, as well as supporting tissue typing services, and are increasingly involved in the clinical development of a pipeline of cell and gene therapies, working in partnership with academia, small and medium‑sized enterprises (SMEs) and global pharmaceutical companies. These NHS organisations provide diverse specialist services and infrastructure and can act as an incubator for companies in need of good manufacturing practice (GMP) production facilities.
One example is the NHS Blood and Transplant (NHSBT) Clinical Biotechnology Centre (CBC),10 which has more than 20 years’ experience of manufacturing molecular therapeutics and has produced over 100 DNA plasmids and recombinant proteins to treat patients across the world, for conditions ranging from arthritis to cancers. Reflecting an increase in demand for their services, CBC is preparing to move to a state-of-the-art expanded facility based at NHSBT Filton in Bristol, which also houses one of four NHSBT cell therapy manufacturing facilities.11 The new facility will host the recently announced NHSBT Gene Therapy Hub.12 Backed by funding from LifeArc and the Medical Research Council (MRC), with support from the Biotechnology and Biological Sciences Research Council (BBSRC), the NHSBT Gene Therapy Hub will provide viral vector manufacturing, training and support services for academic-led groups seeking production of adeno-associated viral (AAV) and lentiviral (LV) vectors and plasmid DNA at GMP and research-grade qualities. CBC’s current projects include a £1.1 million collaboration with Gyroscope Therapeutics,13 Freeline Therapeutics14 and the Centre for Process Innovation (CPI),15 funded by Innovate UK to develop a shared, scalable manufacturing platform to produce novel AAV gene therapies. Translating gene therapies from the lab into a therapy that can be administered into a patient is a complex and expensive process. This project aims to de-risk the transition to costly GMP manufacture of plasmid DNA for subsequent clinical-grade viral vector production.
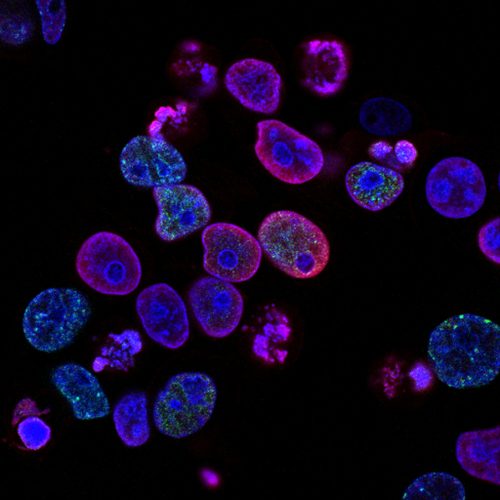

Human colorectal cancer cells treated with a topoisomerase inhibitor and an inhibitor of the protein kinase ATR (ataxia telangiectasia and Rad3 related), a drug combination under study as a cancer therapy. Cell nuclei are stained blue; the chromosomal protein histone gamma‑H2AX marks DNA damage in red and foci of DNA replication in green.
Created by Yves Pommier, Rozenn Josse, 2014.
Successful outcomes from this work will directly benefit patients with conditions such as age-related macular degeneration (AMD) – the leading cause of blindness amongst the elderly for which there is currently no effective treatment for end‑stage disease. As Boris Johnson shared in his first speech as Prime Minister, “it is here in Britain that we are using gene therapy, for the first time, to treat the most common form of blindness”. The FOCUS clinical trial, currently taking place at seven hospitals across England, aims to tackle the underlying genetic cause of AMD and could be effective in halting the disease before people go blind. This gene therapy has been developed by Gyroscope Therapeutics and in February 2019, the first patient was dosed in the FOCUS study to assess safety and biological activity of the gene therapy. This is just one example of innovation in a specific disease area, but serves to demonstrate that the UK is a leader in this field, with Cambridge Enterprise being the top assignee for patents in the AMD gene therapy space (PatSnap).
Another excellent example of UK-led advanced therapy success is chimeric antigen receptor (CAR) T-cell therapy, an innovative immunotherapy approach that involves engineering a patient’s own immune cells to treat their cancer. To engineer the therapy, white blood cells are first collected from the patient by a process known as leukapheresis. The cells are shipped to the manufacturing facility where they are genetically modified to express CARs, which target the patient’s cancer. These engineered ‘CAR-T’ cells are then transported to the treatment hospital and infused into the patient following pre-conditioning chemotherapy. In some instances, CAR T-cell therapy has been curative in patients who had exhausted all other treatment options. To date, NICE has recommended three CAR-T products to be made available to NHS patients: axicabtagene ciloleucel (Yescarta), brexucabtagene autoleucel (Tecartus; both Kite Pharma/Gilead) and tisagenlecleucel (Kymriah, Novartis). NHS England’s commercial deal with Novartis for the indication of acute lymphoblastic leukaemia was the first in Europe and came just days after the therapy was granted European marketing authorisation. This was one of the fastest funding approvals in the 70-year history of the NHS. NHSBT has supported the collection, processing and storage of cells for several of the first wave of NHS treatment centres commissioned to provide CAR T-cell therapy to UK patients. At some centres, cells are collected at NHSBT Therapeutic Apheresis Service16 units and may be cryopreserved at NHSBT Cellular and Molecular Therapies17 laboratories before shipping internationally for manufacture into the CAR T-cell therapy. The final therapeutic product can then be stored by NHSBT under a genetically modified organism (GMO) licence, before being returned to the treatment hospital for administration to the patient. NHS hospital trusts across the UK are also supporting these services, with additional centres now preparing to offer this innovative treatment to patients.
Unsurprisingly, there has been an explosion in IP activity in CAR T-cell therapy, with over 5,600 patents filed in the CAR T area, with year‑on-year filing growth from around 2011.4 The importance of academia and health research institutes in these efforts are clear, with the University of Pennsylvania, UCL Business and the US Department of Health being in the top 10 patent assignees for this technology. Promisingly, the UK appears to be punching above its weight, with well over eight percent of CAR T cell patent filings being made at the UK Intellectual Property Office, ahead of the number of filings being made at the European Patent Office, Korean Patent Office and Australian Patent Office, among others.
Close collaboration between healthcare, academic and commercial organisations is strengthening the UK’s position as a leader in ATMPs”
Patenting advanced therapy technologies can come with challenges, but opportunities for patent protection often arise in a variety of ways during R&D, including improved production and quality control methods, artificial intelligence (AI) and machine learning (ML)-driven analytics and digital health platforms, as well as pharmaceutical products and diagnostic kits. A relevant example is a method patented by NHS Blood and Transplant for the in vitro preparation of adult red blood cells and blood compositions from stem cells, using certain transcription factors for use in medicine, transfusions and transplants.18 The RESTORE trial will explore the recovery and survival of these manufactured red blood cells compared with standard donated red blood cells in healthy volunteers.19 Several patents have been granted in the UK to protect a wide range of advanced therapies, including cellular therapies like stem cell-based medicinal products, gene therapies such as CAR T and AAVs, and tissue-engineered therapies such as live tissue grafts. Building a patent portfolio around new inventions can be a hugely effective strategy to facilitate investment with layers of protection covering new compositions – and new uses for such compositions. In addition to patents, there is also a substantial amount of technical know-how surrounding the production of such therapies, particularly to the standard required for regulatory approval. Know-how may include particular manufacturing steps, quality control, assay techniques, patient monitoring, patient selection algorithms, product design processes or indeed any steps that are performed that cannot be reverse-engineered from publicly available products.
While this type of information may not be protectable through a patent, due to not meeting the novelty or inventive step bar, for example, it is still highly important technical knowledge that is required for production of a medicinal product, and therefore may need to be licensed alongside the patent rights for a particular technology. Steps should be taken to protect such knowledge, including treating the information as confidential in interactions with internal and external stakeholders, including licensing partners. Know-how can have particular value where a patent would be difficult to police, such as if the invention was performed in private clinics where it would be challenging to identify the processes being used. While patent claims to methods also inherently protect the products directly obtained from the method under European patent law, this can be a complicated area where using know-how to protect the invention can also be useful. For example, know‑how can be useful where there are concerns that a process might be performed by a competitor in a country where patent rights are not readily enforceable, and then the product resulting from this process imported and further modified after the steps of the process. One possible example of this is the production of a cellular therapy, where cells derived from an original product might not be considered “directly obtained” by the process of making that original product. In addition, although it is possible to protect a compound or composition for use in treating a disease, it is not possible to obtain patent protection in Europe for methods of treatment on the human body; therefore, if the invention strictly relates to such a method of treatment, where there is not a manufactured product, then know-how could be used to protect this method in Europe.
Repurposing of known therapies can often provide useful patents to bolster an IP portfolio around a protected composition or formulation. This has become particularly relevant as companies pivot their existing therapies and diagnostic methods to treat COVID-19 patients, particularly those that go on to develop severe disease. Researchers at Queen’s University Belfast have recently completed recruitment of patients to a UK-wide clinical trial that is assessing the safety of an innovative cell therapy for COVID-19 patients. The REALIST COVID-19 trial is funded by the Wellcome Trust to investigate the use of mesenchymal stromal cells in the treatment of COVID-19 patients with acute respiratory distress syndrome (ARDS). These cells have been shown in experimental models to reduce inflammation and improve the repair of injured tissue. The therapy is derived from umbilical cord tissue supplied by the NHS Cord Blood Bank (managed by NHSBT) and is being manufactured under MHRA licence by NHSBT’s Cellular and Molecular Therapies team, having been developed by Orbsen Therapeutics. Unsurprisingly, it is expected that there will be an explosion of IP filings surrounding COVID-19, which will start to be published in the next 18 months, and that the value of the IP behind such developments will drive innovation, hopefully resulting in numerous successes in the fight against COVID-19.


Aside from the well documented litigious activities in the pharmaceutical patent area, patents can offer far more than this and can be a useful tool to facilitate commercialisation, including where IP has arisen in, or has been supported by, the NHS. An example of the collaborative approach that can be taken to commercialise an advanced therapy technology is shown by Azellon Cell Therapeutics, which has developed a biological scaffold with mesenchymal stem cells called the Cell Bandage, shown to be useful in the repair of meniscus tears. The IP was developed at the University of Bristol, with the NHS Blood & Transplant facility in Speke, Liverpool, contributing to the manufacturing process. The Cell Bandage technology was spun out from the University of Bristol into Azellon Cell Therapeutics, a commercial company that has obtained funding from Innovate UK and further investment from several backers.
There can be challenges in filing patents in these fast-moving areas. While there is a need to file a patent application before the public disclosure of this information, there is inevitable pressure on researchers to publish their findings in peer‑reviewed journals, as well as share these with funding and clinical trial bodies. There is also a requirement in the patent system in Europe for the invention that the patent is seeking to protect to be made plausible in the application, as filed. Thus, while a patent application could theoretically be filed on the basis of very limited preliminary data, the more data that is available to support the plausibility of the invention, the better.
There are also inherent limitations on patenting of some technologies relating to modification of the human germline and those involving embryos. Seeking the advice of a chartered patent attorney is highly recommended in order to navigate these challenges and to work out the best strategy for patent protection.
It will be exciting to see how the advanced therapy field develops over the coming years”
Another key aspect to consider is ownership. Under UK law, as with many other countries (including in Europe), if an employee is reasonably expected to invent (ie, they work in an R&D role) and the invention arises as part of their usual or assigned duties, then the invention will belong to the employer. However, that is not to say that employees will not be rewarded for their invention, especially if the invention is a huge commercial success for that employer. This includes the NHS, where inventors employed by the NHS should expect to be offered a share in the benefit according to the specific policy of the NHS Trust or the Primary Care Trust, if the invention is of outstanding benefit. It is essential to carefully negotiate in the case of collaborative research, and it is always recommended to have a clear framework in place at the outset that prescribes ownership of IP in the event of an invention.
Close collaboration between healthcare, academic and commercial organisations is strengthening the UK’s position as a leader in ATMPs. There is a huge scope for investment and employment in the UK across manufacturing, logistics and clinical delivery, with potential to bring significant healthcare benefits to patients, and this is at least partly underpinned by the vast IP being generated in this sector. As such, it will be exciting to see how the advanced therapy field develops over the coming years, particularly as the UK continues to be at the forefront of this revolutionary therapeutic area.
About the authors


Rachel Bell is a Trainee Patent Attorney with leading intellectual property firm Marks & Clerk. She has a BSc in Microbiology; worked as a research scientist at Dstl, Ministry of Defence; and undertook a PhD at the Centre for Inflammation Biology and Cancer Immunology at King’s College London focussing on keloid scarring in collaboration with GlaxoSmithKline.


Ceri Roberts is Scientific Training Manager – Cellular and Molecular Therapies at NHS Blood and Transplant. She is an HCPC registered Clinical Scientist with a BSc in Molecular Cell Biology, MSc in Medical Immunology, MRes in Biomedical and Translational Science and a PhD in Immunology, and is involved in the development of education and training to support the clinical delivery of novel advanced cell and gene therapies to NHS patients.
References
- Leading Innovation | The UK’s ATMP Landscape [Internet]. Bioindustry.org. 2019 [cited June 2021]. Available from: https://www.bioindustry.org/resource-listing/leading-innovation.html
- Cell and gene therapy clinical trials [Internet]. Ct.catapult.org.uk. 2021 [cited June 2021]. Available from: https://ct.catapult.org.uk/clinical-trials-database
- CGT Catapult Annual Review 2019 | Accelerating patient access to advanced therapies [Internet]. Ct.catapult.org.uk. 2019 [cited June 2021]. Available from: https://ct.catapult.org.uk/news-media/general-news/press-release-annual-review-2019…
- https://www.patsnap.com/
- https://www.theattcnetwork.co.uk/
- https://www.cytivalifesciences.com/en/gb
- https://orbsentherapeutics.com/
- GE Completes First Logistics Trial of Chronicle for Cell & Gene Therapy [Internet]. GEN – Genetic Engineering and Biotechnology News. 2019 [cited June 2021]. Available from: https://www.genengnews.com/topics/bioprocessing/…
- Advanced Therapy Treatment Centre network awarded £9.5m further investment • ATTC Network – Advanced Therapy Treatment Centre [Internet]. ATTC Network – Advanced Therapy Treatment Centre. 2020 [cited June 2021]. Available from: https://www.theattcnetwork.co.uk/news/advanced-therapy-treatment-centre-network…
- https://www.nhsbt.nhs.uk/clinical-biotechnology-centre/
- https://www.nhsbt.nhs.uk/cellular-and-molecular-therapies/…
- MRC and BBSRC create £18m network of gene therapy hubs to advance promising research into new treatments for patients – LifeArc [Internet]. LifeArc. 2021 [cited June 2021]. Available from: https://www.lifearc.org/news/2021/new-network…
- https://www.gyroscopetx.com/
- https://www.freelinetx.com/
- https://www.uk-cpi.com/
- https://www.nhsbt.nhs.uk/what-we-do/diagnostic-and-therapeutic-services/therapeutic-apheresis/
- https://www.nhsbt.nhs.uk/cellular-and-molecular-therapies/
- Patent number: 9951350
- RESTORE [Internet]. Clinical Trials Unit – NHS Blood and Transplant. 2021 [cited June 2021]. Available from: https://www.nhsbt.nhs.uk/clinical-trials-unit/current-trials-and-studies/restore/
Issue
Related topics
Biologics, Drug Development, Drug Discovery, Drug Manufacturing, Drug Targets, Gene therapy, Genomics, Good Manufacturing Practice (GMP), Industry Insight, Regulation & Legislation, Research & Development (R&D), Technology, Therapeutics, Viruses
Related organisations
Advanced Therapy Treatment Centre (ATTC) network, Biotechnology and Biological Sciences Research Council (BBSRC), Cancer Drugs Fund, Cell and Gene Therapy Catapult (CGTC), Centre for Process Innovation (CPI), Cytiva, European Patent Office (EPO), Freeline, Innovate UK, LifeArc, Medical Research Council (MRC), NHS Blood and Transplant (NHSBT) Cellular and Molecular Therapies, NHS Blood and Transplant (NHSBT) Clinical Biotechnology Centre (CBC), NHS Blood and Transplant (NHSBT) Gene Therapy Hub, NHS England, UK National Health Service (NHS), UK National Institute for Health and Care Excellence (NICE), UK Research and Innovation (UKRI), University College London (UCL), University of Bristol, University of Pennsylvania, US Department of Health & Human Services (HHS)
Related drugs
Related people
Related diseases & conditions
age-related macular degeneration (AMD), Cancer, Coronavirus, Covid-19









