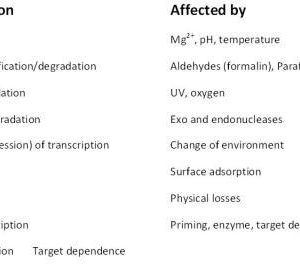
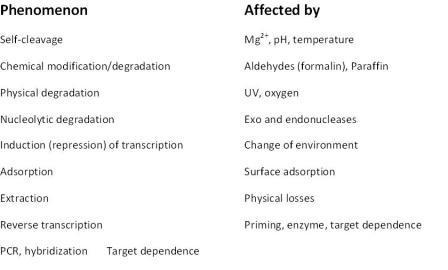
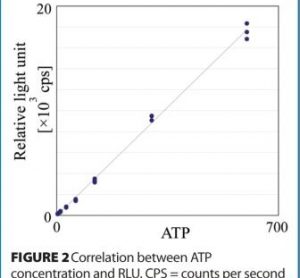
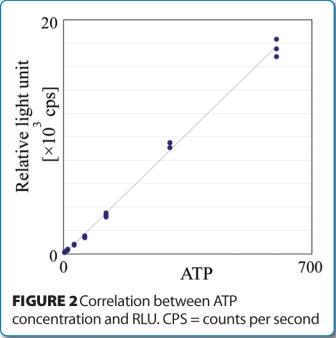
Found in translation
18 December 2012 | By Theresa LaVallee, Senior Director, Translational Medicine Oncology, MedImmune
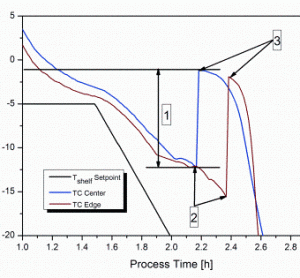
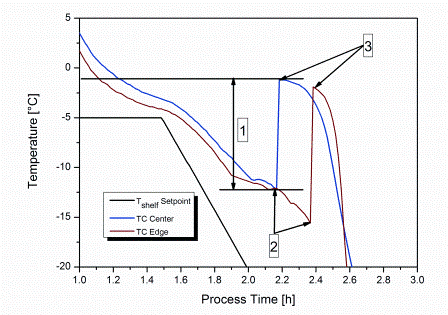
The development of biologic therapies has significantly shifted the treatment paradigm in medicine, particularly in oncology. Groundbreaking research over the past 20 years has sparked new ways of thinking about disease pathology and opened the door for novel targeted therapies that have truly revolutionised medical care. In more recent years,…