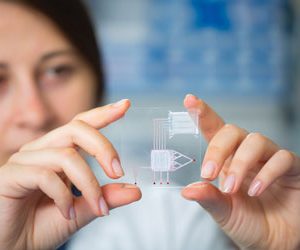
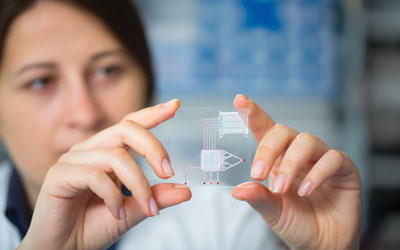
13 December 2011 | By Andrew A. Parsons, Vice President Preclinical Drug Development, GlaxoSmithKline and Steve Street, Vice President, Head of Research Centres of Emphasis, Head of WRD Continuous Improvement, Pfizer and William Strohl, Vice President of Biologics Research, Centocor R&D, a division of Johnson & Johnson Pharmaceutical Research & Development and Eckhard von Keutz, Senior Vice President, Head Global Early Development, Bayer HealthCare
External economic pressures have been identified as the major driver for the pharmaceutical outsourcing market. Over and above the fiscal advantages of adopting this strategy, what other benefits and indeed risks do you see associated with this approach? Steve Street: We definitely began our out - sourcing efforts based on…