List view / Grid view
Manufacturing
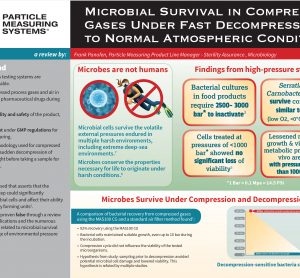
Poster: Microbial survival in compressed gases under fast decompression to normal atmospheric conditions
This scientific poster reviews numerous scientific literature related to microbial survival under a broad range of environmental pressure conditions, and the resilience of microorganisms under variable compression and decompression environments.
Poster: Prevention of dose dumping in PVA-based directly compressed sustained-release matrix tablets
This poster investigates the influence of a directly compressible polyvinyl alcohol (PVA) tablet matrix on the in-vitro drug release that occurs in media of various pH values or alcohol concentrations...
Outsourcing: Current trends in the CDMO sector
Patheon’s Joe Principe examines current trends in outsourcing, highlighting the importance of investment in CDMO partnerships...
Application note: Rapid identification of illicit drug substances using thermal desorption coupled with a portable toroidal trap GC/MS system
This study describes the injection, separation, and identification of 16 drugs compounds in less than 10 minutes using portable gas chromatograph-toroidal ion trap mass spectrometry (PerkinElmer, Torion® T-9 Portable GC/MS) combined with a coiled-wire-filament (CWF) sampling injector to provide an effective tool for onsite analysis of illicit drugs substances...
Whitepaper: Extractable and leachables studies – designed and performed to meet all intended needs
Following FDA legislation in 1999, scrutinising packaging standards for extractables and leachables has become the norm. In this whitepaper, Eurofins examines the ways in which the evaluation of final packaging components is now carried out including the role of liquid and gas chromatography-mass spectrometry in the process.
Poster: Combining spectroscopy and critical process parameters for monitoring continuous tablet manufacturing via multivariate data analysis
CAMO Software discuss how combining spectroscopy and critical process parameters can assist in monitoring continuous tablet manufacturing...
Fujifilm Diosynth Biotechnologies announce successful completion of its 100th paveway
Fujifilm Diosynth Biotechnologies has announced the successful completion of its 100th program using its pAVEway™ Advanced Protein Expression system...
Poster: Optimised purity and recovery of a monoclonal antibody using mixed-mode chromatography media
Bio-Rad discuss the technique of optimising the purity of a monoclonal antibody using mixed-mode chromatography media...
Metrohm & CAMO Software are announcing a global partnership
Optimising analysis in the laboratory with modern instruments and high-performance software...
DiaSorin and Tecan to collaborate in new platform development
DiaSorin and Tecan Group have announced that they have agreed to collaborate in a development under which DiaSorin will make use of Tecan’s Fluent® Laboratory Automation Solution as its nucleic acid extraction platform...
CAMO releases new approach to modeling and monitoring time-dependent processes: Batch Modeling for the Unscrambler® X software suite
CAMO Software announced the release of Batch Modeling, a new add-on to their all-in-one Multivariate Data Analysis (MVA) and process monitoring software suite Unscrambler® X...
Guide To Testing Services
Welcome to European Pharmaceutical Review’s Guide to Testing Services, the second in our new series of ‘Guide to …’ supplements. In this edition, five leading testing service suppliers explain how their service offering meets current industry needs...
Separation and purification applications for mutagenic impurities
Classically, isolation of key intermediates and the resultant active pharmaceutical ingredient (API) has formed the basis of clean up and purification strategies within the pharmaceutical industry...
Personalised medicine, batch size of ONE, the new challenge to fill-finish?
Wenzel Novak PhD gives some considerations on container, environment, process and automation for small batch sizes in the downstream process of fill-finish…