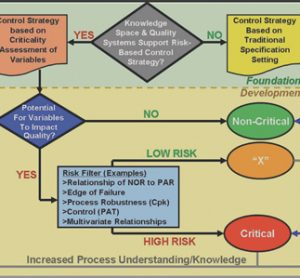
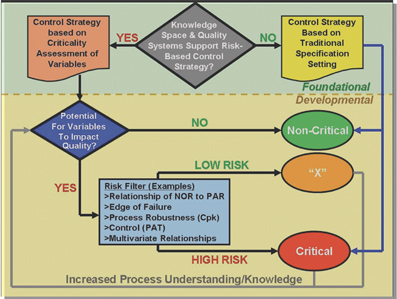
Siemens: innovation, industry and implementation
19 June 2008 | By
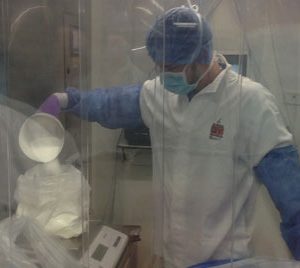
Siemens seeks to deliver breakthrough innovations to give customers a unique competitive edge, in turn enabling societies to master their most vital challenges and creating sustainable value. Siemens was one of the first suppliers to adopt the new guidelines of the Food & Drug Administration (FDA) and the European Agency…