Environmental Monitoring In-Depth Focus 2016
24 August 2016 | By European Pharmaceutical Review
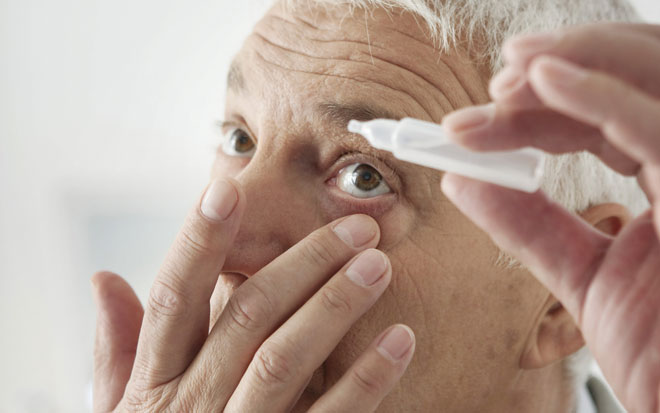
In this Environmental Monitoring In-Depth Focus: Bacterial endotoxin contamination and testing limits in ophthalmics; The basics of environmental monitoring in aseptic units; Environmental Monitoring Roundtable...