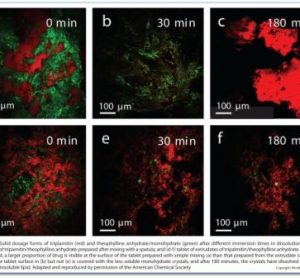
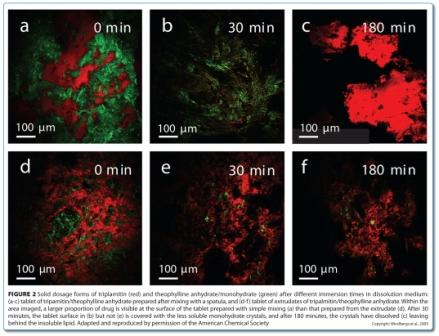
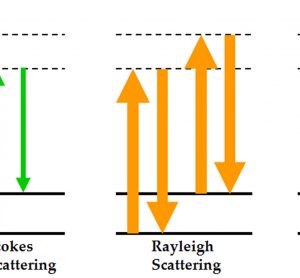
Applications of Raman, CARS and SRS imaging in dosage form development
26 April 2012 | By Clare Strachan, Senior Lecturer Pharmaceutical Sciences, School of Pharmacy, University of Otago
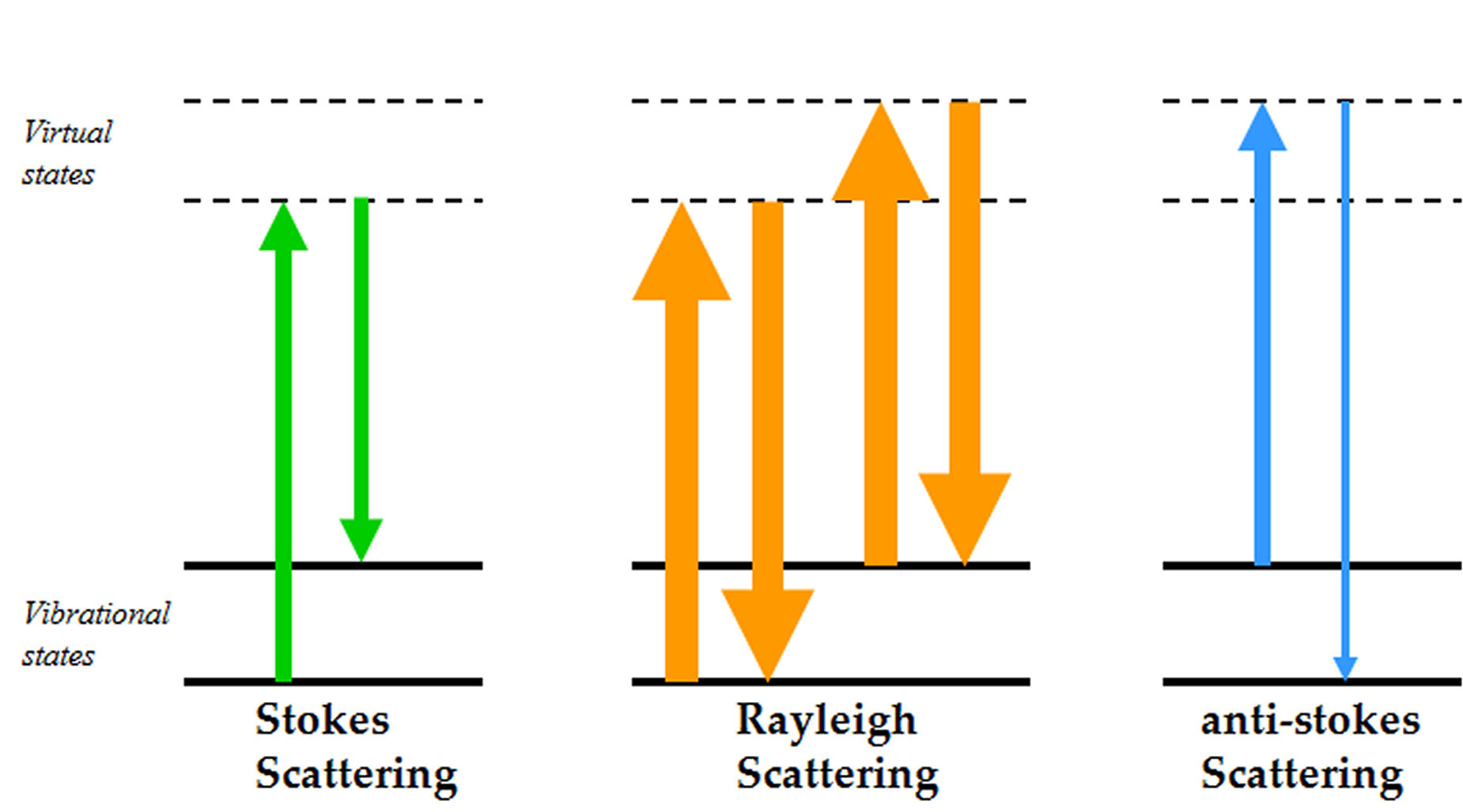
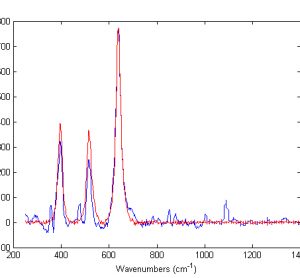
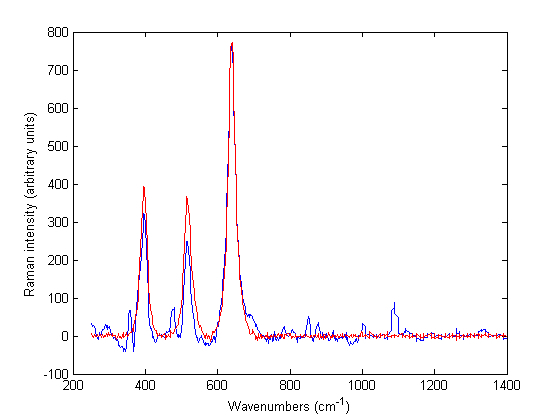
The use of Raman spectroscopy in pharmaceuticals has grown enormously since its appearance on the scene in the 1980s1-4. While typical Raman spectroscopy setups are able to provide chemical and physicochemical information about the sample on the bulk level, most solid samples in the pharmaceutical setting may not be assumed…