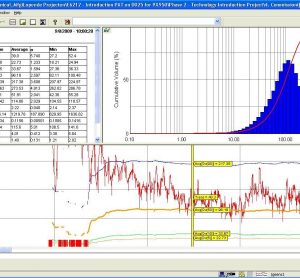
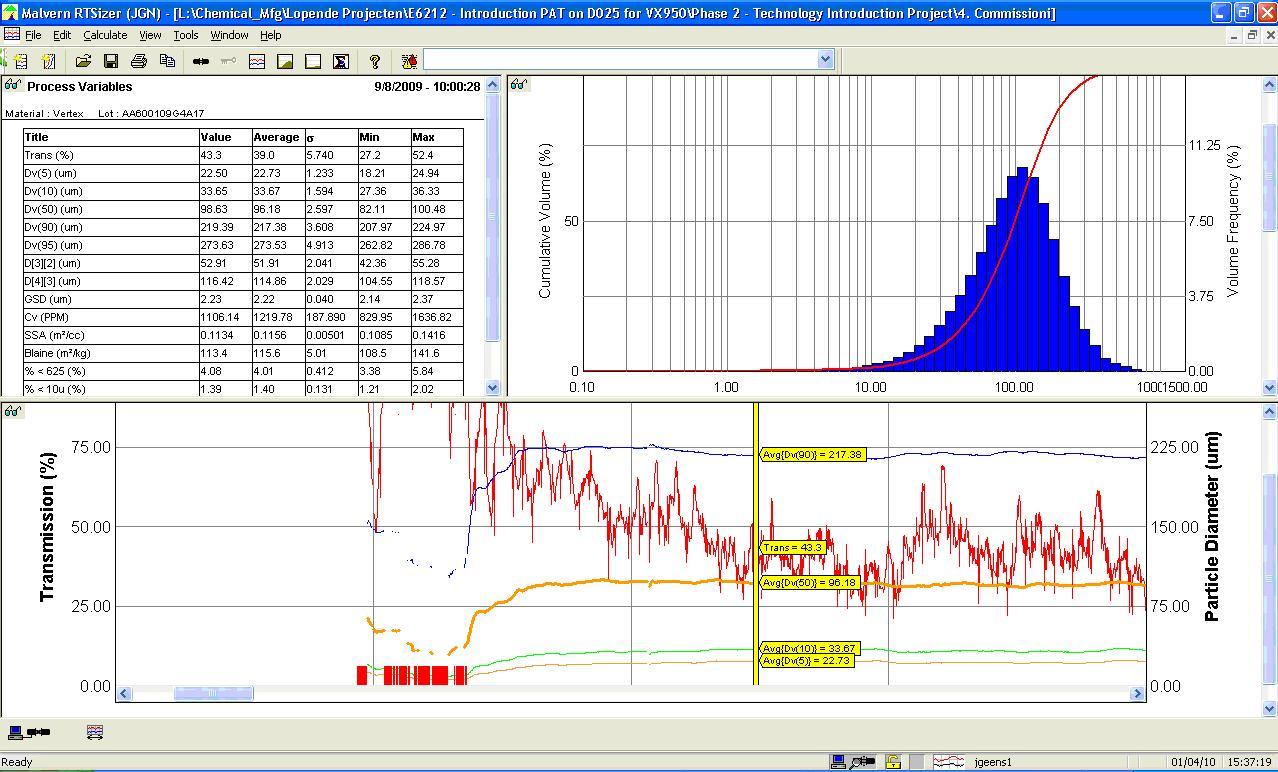
Process Analytical Technology (PAT) In-Depth Focus 2012
In this PAT In-Depth Focus: Process analytics experiences in biopharmaceutical manufacturing; Correlation between powder rheology data and processability in solid dosage form manufacturing; Expert industry users of process analytical technologies for pharmaceuticals pose questions for leading vendor experts of process analytical technology...