Whitepaper: The importance of data integrity in current regulation
Posted: 12 December 2018 | Particle Measuring Systems | No comments yet
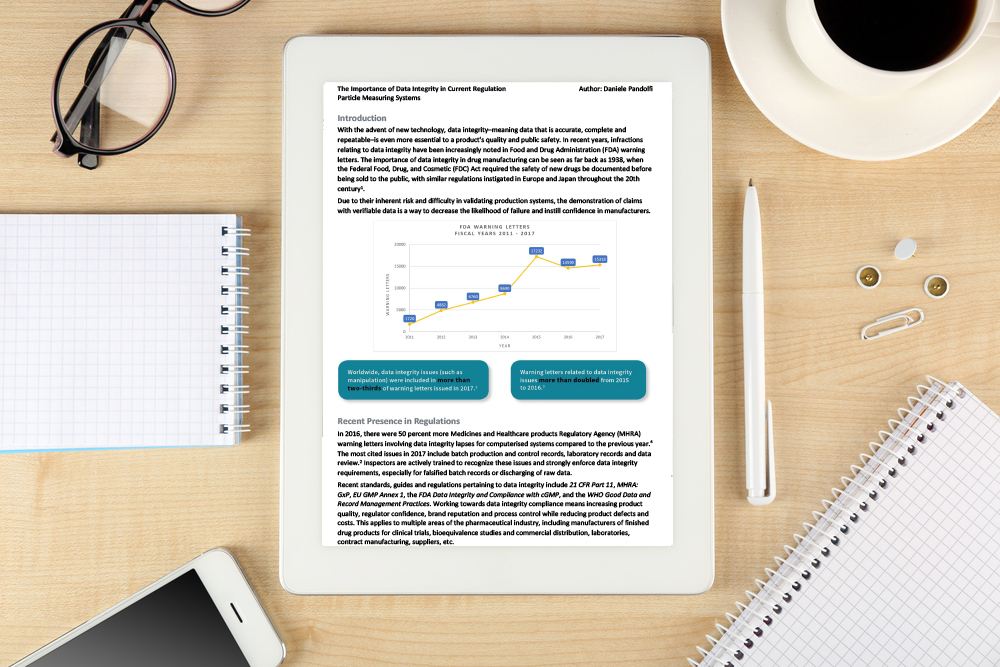
With the advent of new technology, data integrity−meaning data that is accurate, complete and repeatable−is even more essential to a product’s quality and public safety.
In recent years, infractions relating to data integrity have been increasingly noted in Food and Drug Administration (FDA) warning letters. The importance of data integrity in drug manufacturing can be seen as far back as 1938, when the Federal Food, Drug, and Cosmetic (FDC) Act required the safety of new drugs be documented before being sold to the public, with similar regulations instigated in Europe and Japan throughout the 20th century 1.
Due to their inherent risk and difficulty in validating production systems, the demonstration of claims with verifiable data is a way to decrease the likelihood of failure and instill confidence in manufacturers.
The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related content from this organisation
Issue
Related topics
Data Analysis, Drug Safety, QA/QC, Regulation & Legislation, Technology