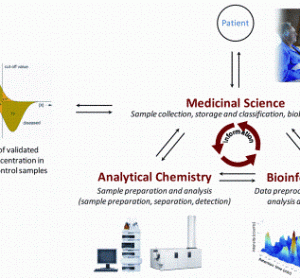
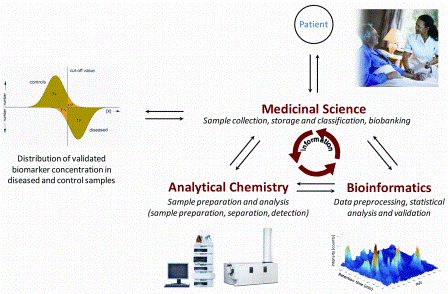
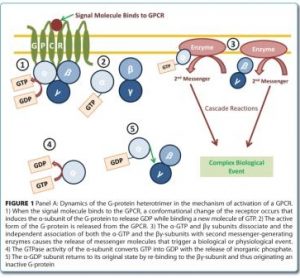
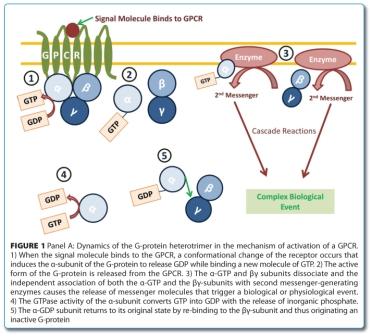
Discovery and validation of protein biomarkers
10 July 2012 | By Péter Horvatovich & Rainer Bischoff, Analytical Biochemistry, Department of Pharmacy, University of Groningen
Biomarkers are biological characteristics that are objectively measured and evaluated as indicators of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention. Biomarkers can be used to determine disease onset, progression, efficacy of drug treatment, patient susceptibility to develop a certain type of disease or predict efficacy…