Nanotechnology tackles problems with noninvasive glucose monitoring
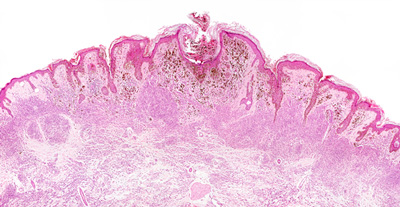
For diabetes mellitus patients who treat themselves with insulin, the need to self-monitor blood glucose in order to establish their insulin dose is a major burden, and highlights the need to develop non-invasive glucose measurement technologies. Up until now, such non-invasive systems that also have adequate measurement performance under daily…