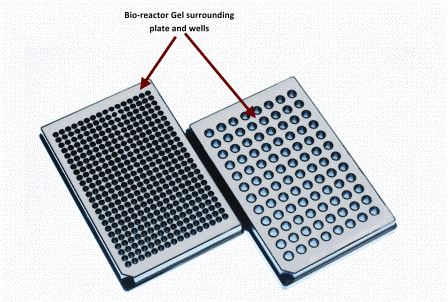
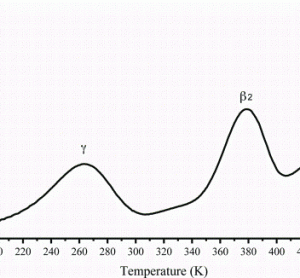
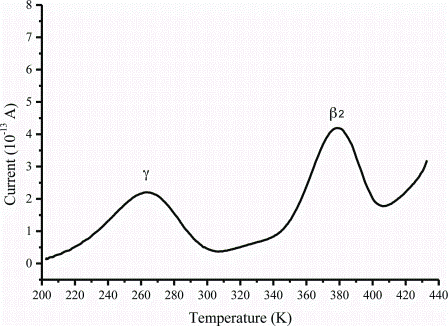
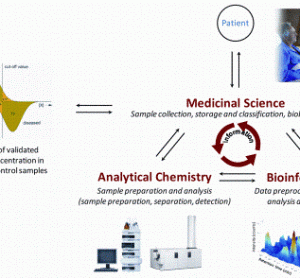
Applications of MRI to controlled drug delivery devices
22 October 2012 | By Mick Mantle, Department of Chemical Engineering & Biotechnology, University of Cambridge
Magnetic resonance imaging (MRI) is a technique that is traditionally used as a diagnostic clinical imaging tool. However, there are now an increasing number of non-medial applications where MRI has seen unrivalled success. One of those areas is in its application to pharmaceutical research. The aim of this article is…