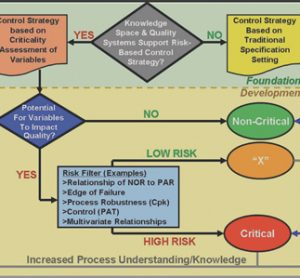
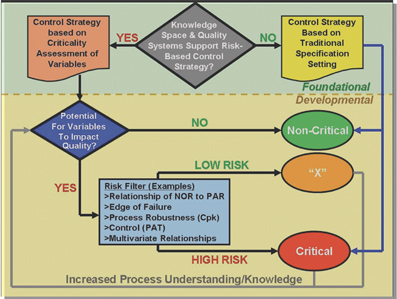
Quo vadis, Laboratory Automation Education?
7 April 2008 | By
The increasing need for improved efficiency, precision and 24/7 operation imply more and more sophisticated measures in laboratory automation. This is true for a variety of fields – from pharmaceutical to food, agricultural, and the petrochemical industry, as well as forensics and medical diagnostics. Chemical and biological tests have to…