The central role of excipients in drug formulation
18 April 2013 | By Pascal Furrer, Pharmacist
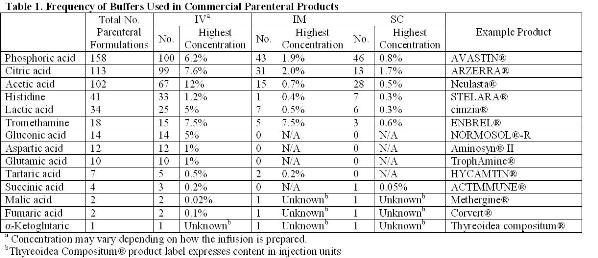
Active substances are rarely administered alone. For example, levothyroxine, a synthetic form of the thyroid hormone, indicated in the treatment of hypothyroidism, is administered at a very low dosage, ranging from 15 μg to 200 μg. These very small amounts of powder mean that it is not possible to manufacture…