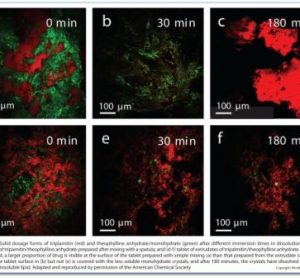
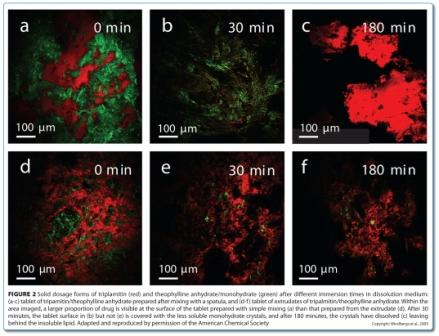
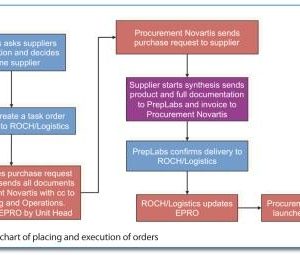
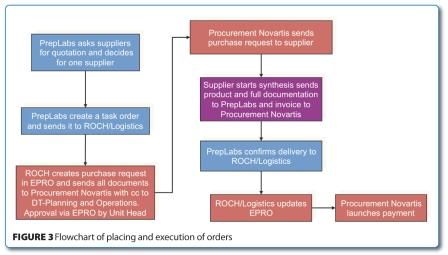
At the crossroads: The next decade
26 April 2012 | By J. Paul Robinson, Purdue University Cytometry Laboratories & Weldon School of Biomedical Engineering, Purdue University; Bernd Bodenmiller, Group leader, Institute of Molecular Life Sciences, University of Zurich; Valery Patsekin and Bartek Rajwa, Purdue University Cytometry Laboratories, Purdue University; and V. J. Davisson, Medicinal Chemistry & Molecular Pharmacology, Purdue University
Flow cytometry is the technology that has the most impact on single-cell analysis. Over the past 40 years, it has arguably been the single most important research technique in the fields of basic and applied immunology. Flow cytometry excels in quantitative evaluation of receptor expression, separation of functionally defined cell…