New purification method could enhance protein drug manufacturing
Posted: 1 March 2023 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
Bioconjugate-functionalised nanoparticles can isolate proteins from a bioreactor fast and inexpensively, MIT researchers have found.


In a recent study, Massachusetts Institute of Technology (MIT) researchers developed a new purification method to cut the costs of manufacturing protein drugs. The engineers used bioconjugate-functionalised nanoparticles to quickly and inexpensively isolate proteins from a bioreactor.
Producing biologics by isolating proteins
Biologics like antibodies and other protein-based drugs are produced by living cells such as yeast in large bioreactors.
Once generated, the proteins need to be isolated from the reactor. Chromatography is the standard method. It separates proteins based on their size. However, the process can take up half of the total manufacturing cost.
Crystallising proteins is considered too slow for industrial use and doesn’t work well at low concentrations of protein. To overcome those obstacles, Varanasi’s lab decided to adapt nanoparticles so that they could locally increase the protein concentration at the surface. This would also provide a template to allow the proteins to align correctly and form crystals.
The nanoparticles “act as templates for enhancing protein crystal formation at low concentrations,” stated Kripa Varanasi, a professor of mechanical engineering at MIT and the senior author of the study.
Protein crystallisation of bioconjugate nanoparticles
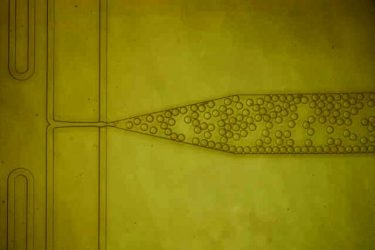

A microfluidic device was designed to combine protein solution with nanoparticles and then form thousands of tiny, identical droplets. Inside each of these droplets, the proteins interact with the nanoparticles, which help them to form protein crystals. (Credit: MIT)
So, Varanasi and his colleagues tried a different approach, based on protein crystallisation.
The researchers demonstrated their method with lysozyme, an enzyme whose crystallisation properties have been well studied, and insulin.
To create the surface required, the team coated gold nanoparticles with bioconjugates maleimide (MAL) and N-hydroxysuccinimide ester (NHS). The authors explained that these are commonly used for tagging proteins for study or attaching protein drugs to drug-delivering nanoparticles.
When solutions of proteins are exposed to these coated nanoparticles, the proteins accumulate at the surface and bind to the bioconjugates, the researchers noted. Furthermore, the bioconjugates compel the proteins to align themselves with a specific orientation, creating a scaffold for additional proteins to come along and join the crystal.
Findings from new purification method
With the coated particles, the researchers saw a sevenfold reduction in the induction time, how long it takes for crystals to begin forming.
A threefold increase in the nucleation rate, how quickly the crystals grow once started, was also observed.
“Even at low protein concentrations, we see a lot more crystals forming with these bioconjugate-functionalised nanoparticles,” Caroline McCue, lead author of the study shared. “The functionalised nanoparticles reduce the induction time so much because these bioconjugates are providing a specific site for the proteins to bind. And because the proteins are aligned, they can form a crystal faster.
”The findings indicated that crystallisation occurred much faster when the proteins were exposed to the bioconjugate-coated nanoparticles, compared to bare nanoparticles or no nanoparticles.
Analysing proteins crystals using machine learning
In addition, the team used machine learning to analyse thousands of images of crystals. McCue explained: “we needed to have a huge dataset to be able to really measure whether our approach was improving the induction time and nucleation rate of crystallisation. With so many images to process, machine learning is the best way to be able to determine when crystals are forming in each image without having to go through and manually count each one.”
The MIT team is now working to scale up the process for use in an industrial bioreactor, and demonstrating that it can work with monoclonal antibodies, vaccines, and other useful proteins.
“This is a general approach that could be scaled to other systems as well. If you know the protein structure that you’re trying to crystallise, you can then add the right bioconjugates that will force this process to happen,” Varanasi stated.
In addition to the Gates Foundation, the research was partly funded by a National Science Foundation Graduate Research Fellowship.
The results were published in ACS Applied Materials & Interfaces.
Related topics
Analytical techniques, Biopharmaceuticals, Bioprocessing, Bioproduction, Chromatography, Drug Manufacturing, Particle Sizing, Protein Crystallisation, protein structure, Proteins, Research & Development (R&D), Technology, Therapeutics









