Spectroscopy milestone signals advance in protein imaging
Posted: 11 January 2024 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
An ultra-high sensitivity and super-resolution infrared imaging technique for single proteins could lead the way to a multitude of applications using infrared nanospectroscopy.

Researchers at the Institute for Molecular Science in Japan have successfully observed vibrational spectra of single proteins using infrared nanospectroscopy. The observations made consisted of ~500 amino acid residues, using advanced measurement techniques based on near-field optical microscopy. Comparatively, this method is a challenge with conventional infrared spectroscopy.
Value of the infrared nanospectroscopy research
The research represents a major advancement towards technological innovations like ultra-sensitive and super-resolution infrared imaging, as well as single-molecule vibrational spectroscopy.
Infrared spectroscopy is a common tool used to analyse the structure and chemical composition of materials via vibrational spectra measurement, the researchers highlighted.
In recent years, there has been increased demand for ultra-high sensitivity and super-resolution infrared imaging, due to rapid advances in nanotechnology. However, the team emphasised that their achievement is important for the field because a single protein cannot be measured even with sensitive infrared microspectroscopy.
However, infrared nanospectroscopy uses light confined to the nanometer scale, and can facilitate detailed analysis of extremely small samples.
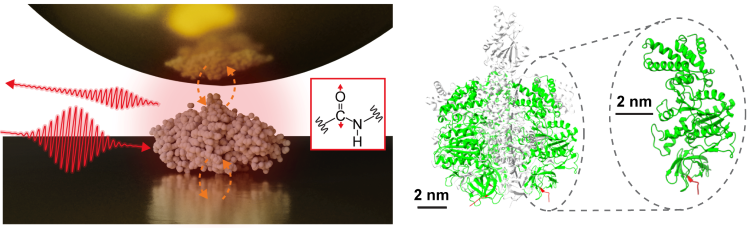

Single protein infrared vibrational spectroscopy. (Left) Scheme of near-field infrared spectroscopy measuring a single protein. (Right) The structure of the protein complex F1-ATPase and the subunit measured in this study. (Credit: Jun Nishida)
Spectroscopy research method
Firstly, by isolating a single protein, a sub-unit comprising a protein complex called F1-ATPase, on a gold substrate, the researchers performed near infrared spectroscopy (NIR) measurements in an ambient environment.
This research milestone may lead to characterisation of local structural organisations of individual proteins, the team noted.
Alongside their observations, Nishida et al. developed a new theoretical framework. This described the nanoscale interactions between the infrared near field and protein.
Using the theory, the researchers successfully quantitatively reproduced the experimental vibrational spectra that they observed.
Ultimately, data from this study holds great value for future chemical analysis of biomolecules, leading the way for a variety of applications using nanoscale infrared spectroscopy.
Results from the experimental study were recently published in Nano Letters.
Related topics
Analytical techniques, Biopharmaceuticals, Data Analysis, Imaging, Industry Insight, Nanoparticles, Near Infrared Spectroscopy (NIR), Proteins, Research & Development (R&D), Spectroscopy, Technology









