Teva’s migraine biologic Ajovy marks phase III progress for paediatric patients
Posted: 16 January 2026 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
The monoclonal antibody could provide a new preventative treatment option for episodic migraines, new late-stage data suggests.
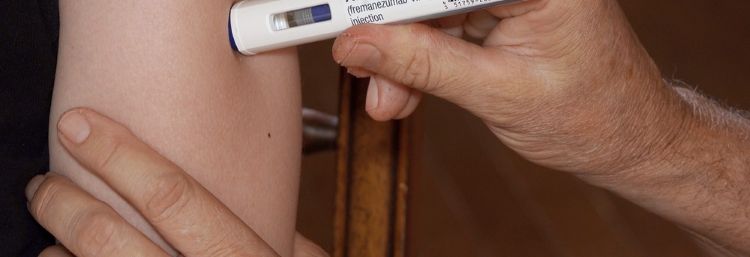

Teva’s Ajovy (fremanezumab-vfrm) has demonstrated potential as the first calcitonin gene-related peptide (CGRP) antagonist treatment option for preventing episodic migraines in paediatric and adult patients.
Data from the phase III SPACE study published in New England Journal of Medicine (NEJM) showed Ajovy significantly reduced monthly migraine days and monthly headache days by 2.5 days compared to 1.4 days with placebo over 12 weeks in children between 6-17 years old.
Additionally, the monoclonal antibody reduced monthly headache days of at least moderate severity by 2.6 days versus 1.5 days for placebo. With Ajovy, 47.2 percent of participants achieved a ≥ 50 percent reduction in monthly migraine days, compared to 27.0 percent with placebo.
The study’s lead author Dr Andrew Hershey said: “The SPACE trial demonstrates that a CGRP-targeted preventive therapy like fremanezumab-vfrm (Ajovy) can significantly reduce the frequency of attacks of migraine in youth, giving physicians critical evidence to guide care for this underserved population.”
The SPACE trial demonstrates that a CGRP-targeted preventive therapy like fremanezumab-vfrm (Ajovy) can significantly reduce the frequency of attacks of migraine in youth”
Ajovy is administered subcutaneously as a single dose in a pre-filled autoinjector or in a pre-filled syringe.
Teva received US approval for Ajovy in August 2025, authorising its use in certain adults and children. The National Institute for Health and Care Excellence (NICE) in March 2020 recommended fremanezumab to prevent chronic migraine in eligible adults.
Dr Eric Hughes, PhD, Executive Vice President, Global R&D and Chief Medical Officer at Teva said: “With an estimated 1 in 10 children and adolescents in the US living with migraine, the need for effective preventive options is critical as this condition can disrupt daily life for patients and families.”
Last December, AbbVie also shared promising phase III findings for its CGRP-targeting migraine therapy. If approved in the EU, the small molecule drug atogepant would provide adults with a new acute treatment option for migraine attacks.
Related topics
Big Pharma, Biologics, business news, Clinical Development, Clinical Trials, Drug Delivery Systems, Drug Development, Drug Safety, Research & Development (R&D), Therapeutics









