Drug Formulation In-Depth Focus 2022
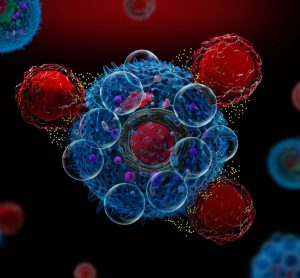
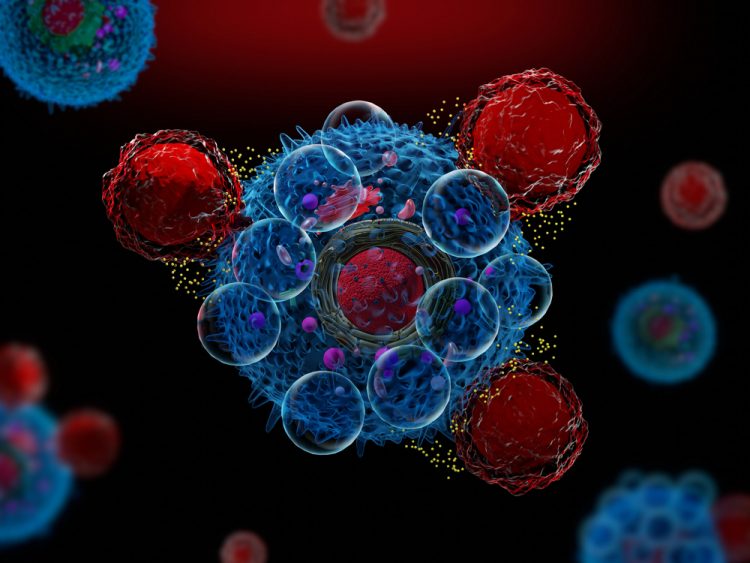
Features in this in-depth focus highlight developments in the MRI contrast agent space, explore the development of precision medicine for chronic diseases and explain why immunotherapies should engage the innate immune system in the fight against cancer.







![Recipharm logo on building in Bordeaux, France[Credit: sylv1rob1/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Recipharm-300x278.jpg)
![Recipharm logo on building in Bordeaux, France[Credit: sylv1rob1/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Recipharm.jpg)