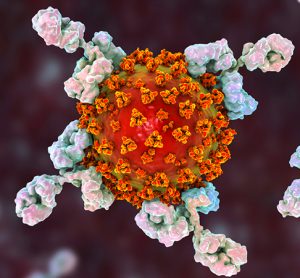
Trial of new HIV PrEP medication stopped early due to effectiveness
The trial was stopped because the data suggested that injections of the long-acting antiretroviral drug cabotegravir (CAB LA) are highly effective for HIV pre-exposure prophylaxis (PrEP) in women.




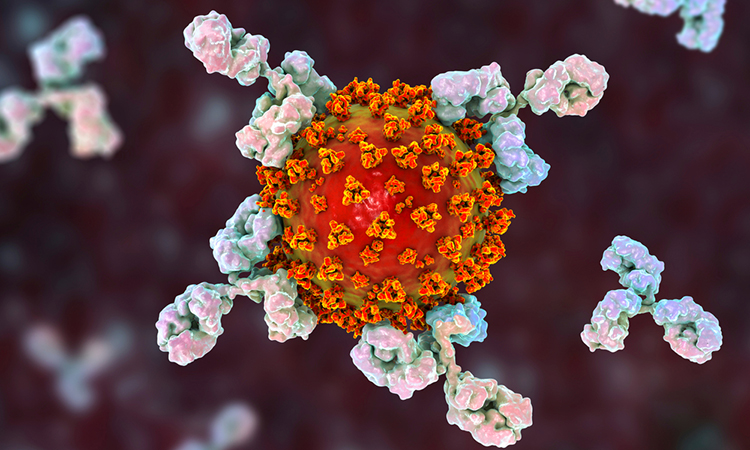
![Artist's depiction of an ultrapotent COVID-19 vaccine candidate in which 60 pieces of a coronavirus protein (red) decorate nanoparticles (blue and white). The vaccine candidate was designed using methods developed at the UW Medicine Institute for Protein Design. The molecular structure of the vaccine roughly mimics that of a virus, which may account for its enhanced ability to provoke an immune response [Credit: Ian Haydon/ UW Medicine Institute for Protein Design].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Nanoparticle-covid-19-vaccine-300x278.jpg)
![Artist's depiction of an ultrapotent COVID-19 vaccine candidate in which 60 pieces of a coronavirus protein (red) decorate nanoparticles (blue and white). The vaccine candidate was designed using methods developed at the UW Medicine Institute for Protein Design. The molecular structure of the vaccine roughly mimics that of a virus, which may account for its enhanced ability to provoke an immune response [Credit: Ian Haydon/ UW Medicine Institute for Protein Design].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Nanoparticle-covid-19-vaccine.jpg)