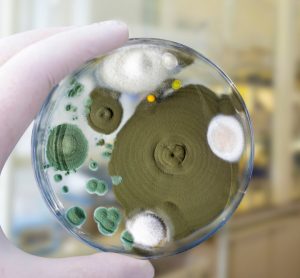
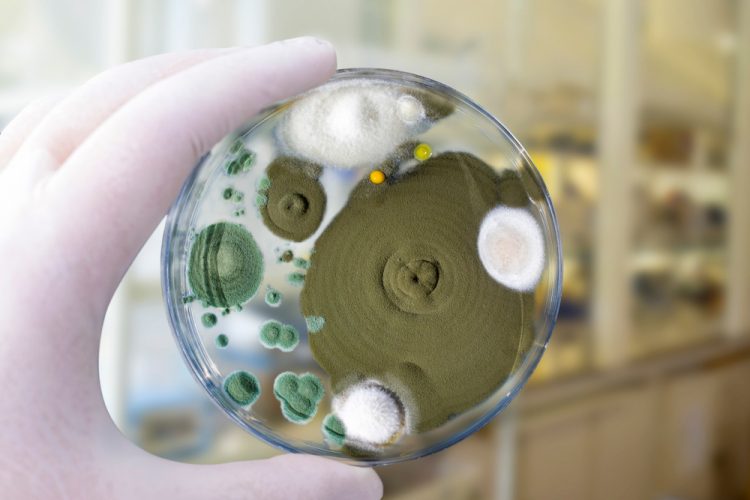
FDA suggests principles for implementing bio-fluorescent particle counters
FDA provides guidance on the validation and regulations for bio-fluorescent particle counting technology as an alternative, continuous method for bioburden testing in air and water systems.



















![MBE Medals [Credit: HCSA (Health Care Supply Association)].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/MBE-300x278.png)
![MBE Medals [Credit: HCSA (Health Care Supply Association)].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/MBE.png)





