Application Note: Quantitation of intact therapeutic protein in plasma matrix by LC/MS
Posted: 8 January 2019 | Sciex | No comments yet
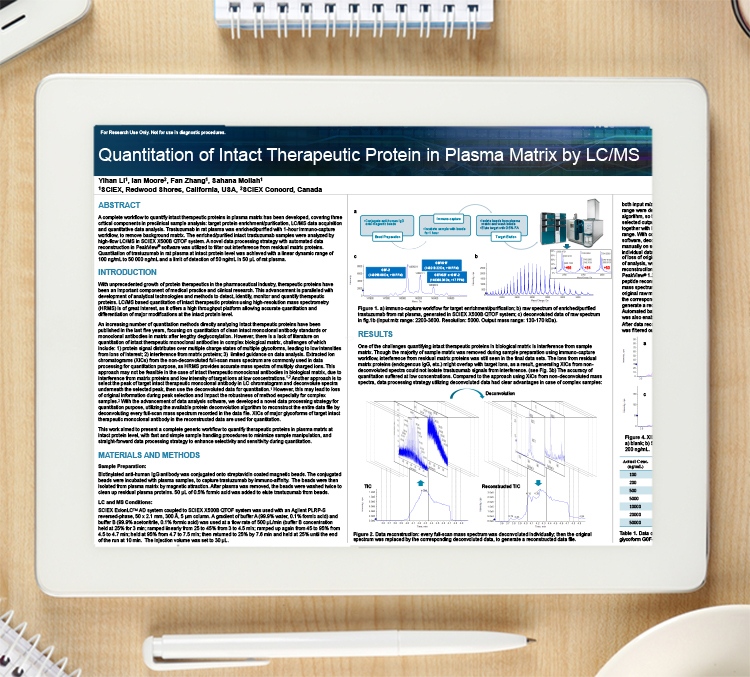
A complete workflow to quantify intact therapeutic proteins in plasma matrix has been developed, covering three critical components in preclinical sample analysis: target protein enrichment/purification, LC/MS data acquisition and quantitative data analysis.
Trastuzumab in rat plasma was enriched/purified with one-hour immuno-capture workflow, to remove background matrix. The enriched/purified intact trastuzumab samples were analysed by high-flow LC/MS in SCIEX X500B QTOF system. A Novel data processing strategy with automated data reconstruction in PeakView® software was utalised to filter out interference from residual matrix proteins. Quantitation of trastuzumab in rat plasma at intact protein level was achieved with a linear dynamic range of 100 ng/mL to 50,000 ng/mL and a limit of detection of 50ng/mL in 50 µL of rat plasma.
Related content from this organisation
- Enhancing nitrosamines analysis: a focus on NDSRIs
- Project worth £3.1mn to develop advanced controls for biopharma manufacturing
- Global mass spectrometry market to value $7.3 billion by 2028
- Expert view: The future of biologics quality – multiple attribute methodology
- Application note: New Peak Detection Using the SCIEX OS Software 1.5 MAM Workflow
Related topics
Analytical techniques, Biologics, Biopharmaceuticals, Data Analysis, Liquid Chromatography - Mass Spectrometry (LC-MS), Mass Spectrometry, Proteins, QA/QC, Therapeutics