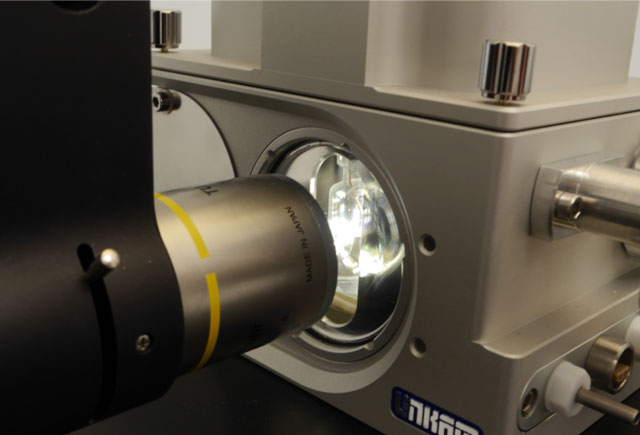
Novel preservation method could improve storage of biologics
Light-assisted drying (LAD) is a new optical processing technique for forming trehalose amorphous solids to preserve biologics, while avoiding the freezing step necessary for lyophilisation.





![PCI Pharma Services logo on sign outside Canadian facility [Credit: JHVEPhoto/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/PCI-Pharma-Services-300x278.jpg)
![PCI Pharma Services logo on sign outside Canadian facility [Credit: JHVEPhoto/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/PCI-Pharma-Services-e1653043421551.jpg)