Bacterial Endotoxin Test using LAL methodology: overcoming interfering factors
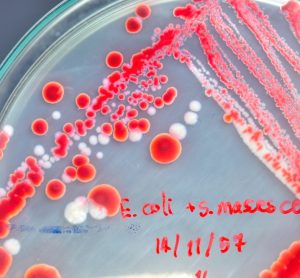
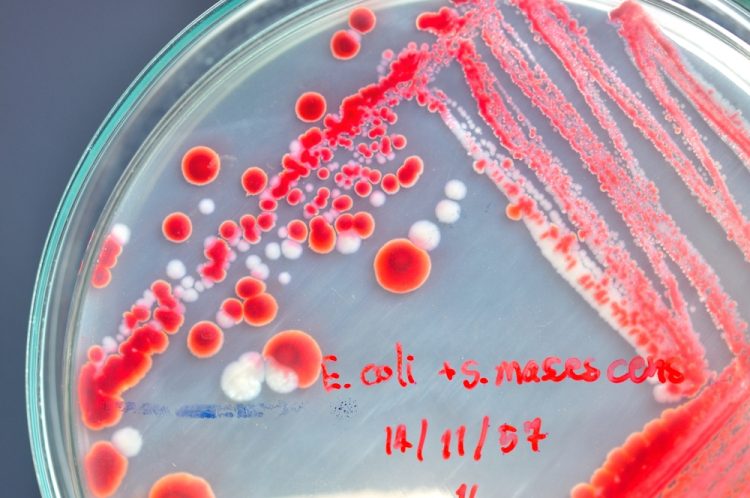
The Bacterial Endotoxin Test, using LAL methodology, is a key in-process and final product release test for sterile pharmaceuticals and medical devices. One of the challenges with LAL methodology is overcoming interfering substances as demonstrated by inhibition or enhancement of an endotoxin challenge. Here, Bio Products Laboratory’s Dr Tim Sandle…