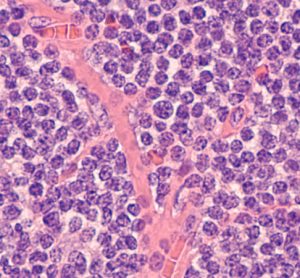
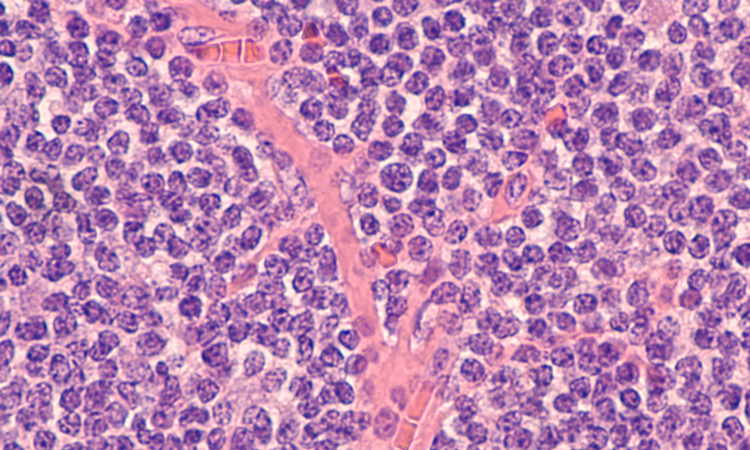
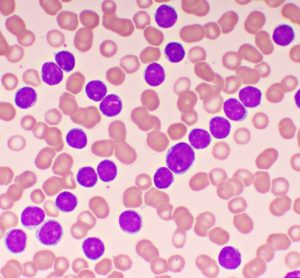
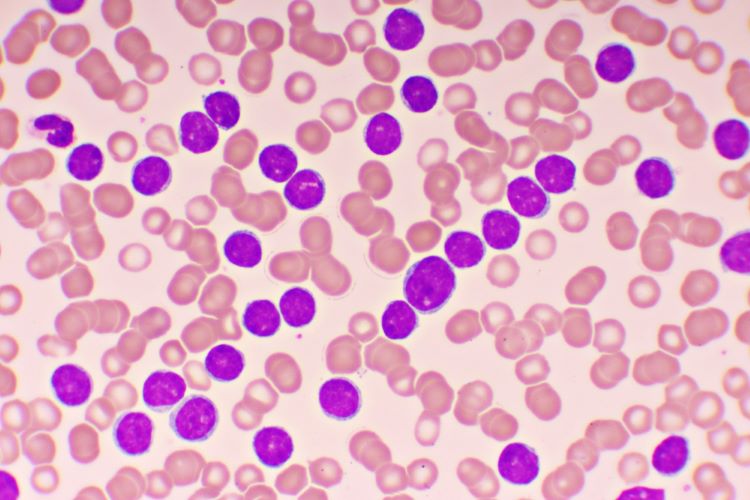
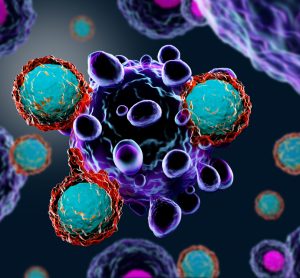
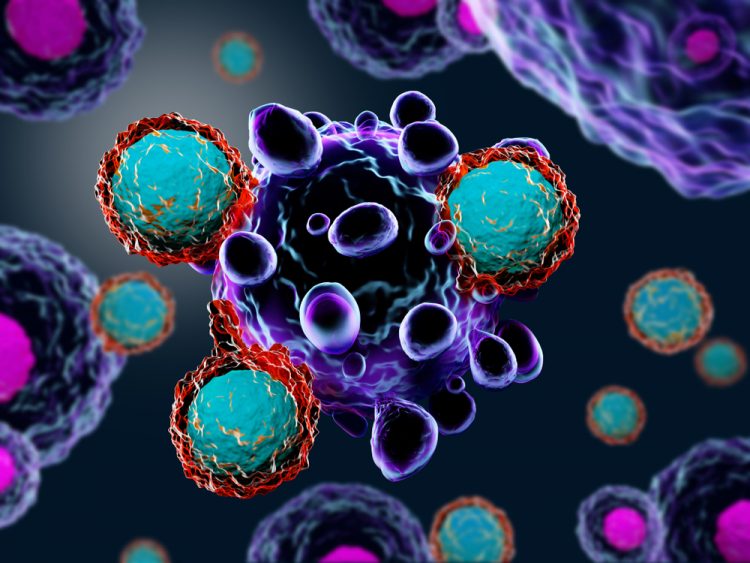
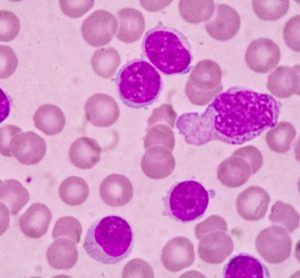
Targeted therapies for chronic lymphocytic leukaemia in an evolving treatment landscape
Dr Mehrdad Mobasher, Chief Medical Officer for Hematology, BeiGene, discusses the evolution of therapies for chronic lymphocytic leukaemia (CLL), the promise of targeted treatments and what could be on the horizon of the therapeutic landscape for this disease.








![sign of the European Medicines Agency building in Amsterdam [Credit: martinbertrand.fr/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/EMA-1-300x278.jpg)
![sign of the European Medicines Agency building in Amsterdam [Credit: martinbertrand.fr/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/EMA-1-e1617017465349.jpg)