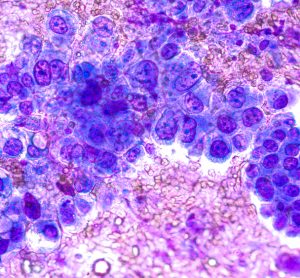
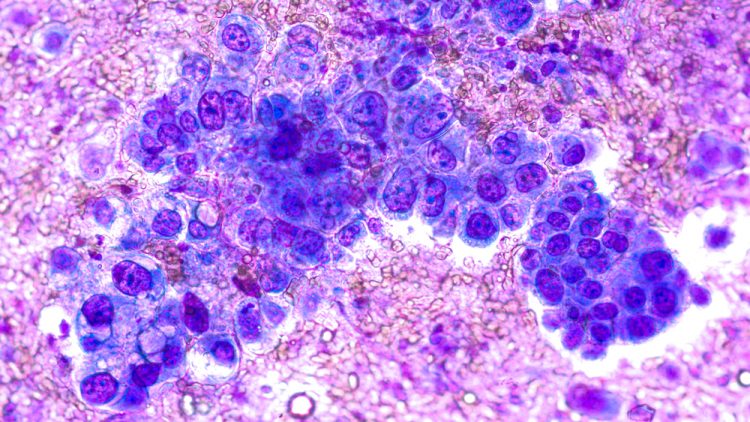
AstraZeneca’s Tagrisso: first medicine approved in the UK under Project Orbis
Tagrisso (osimertinib) was approved for an indication extension after it reduced risk of death by over 80 percent in certain early-stage non-small cell lung cancer patients.
















![A cryomicroneedle patch ready for deployment [Credit: Chang et al. /DOI number: 10.1038/s41551-021-00720-1].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/cryomicroneedle-patch-300x278.jpg)
![A cryomicroneedle patch ready for deployment [Credit: Chang et al. /DOI number: 10.1038/s41551-021-00720-1].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/cryomicroneedle-patch-e1620132484839.jpg)