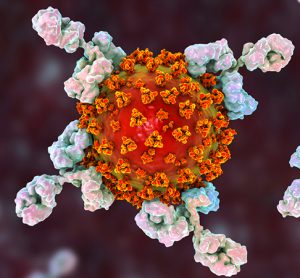
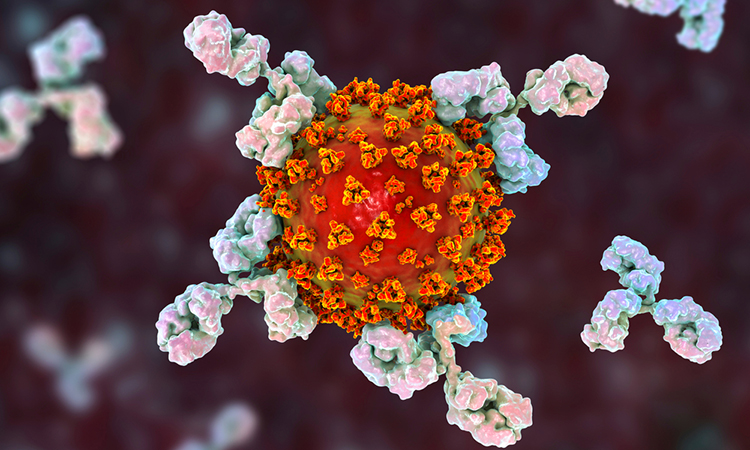
Study finds some antibody therapies are not effective against new COVID-19 variants
In vitro neutralisation assays show REGEN-COV and AZD7442 are effective against the new SARS-CoV-2 variants, while other antibody therapies, including Eli Lilly’s bamlanivimab, were not.





![Vial labelled heparin. [Credit: SamaraHeisz5 / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Heparin-300x278.jpg)
![Vial labelled heparin. [Credit: SamaraHeisz5 / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Heparin-scaled.jpg)