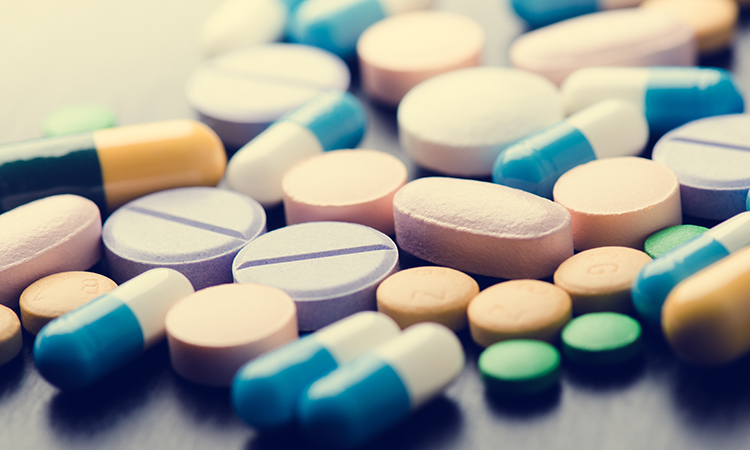
Sourcing of active pharmaceutical ingredients
Considerations when sourcing active pharmaceutical ingredients (APIs) can often be condensed to ones of economy and purity. Here, Dave Elder highlights the importance of defining those attributes that impact final product quality to ensure pharma companies find the best ingredient suppliers.







![US Department of Justice logo on top of an American Flag [Credit: US-Department-of-Justice].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/US-Department-of-Justice-300x278.jpg)
![US Department of Justice logo on top of an American Flag [Credit: US-Department-of-Justice].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/US-Department-of-Justice-e1613474706496.jpg)