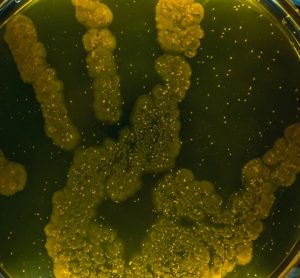
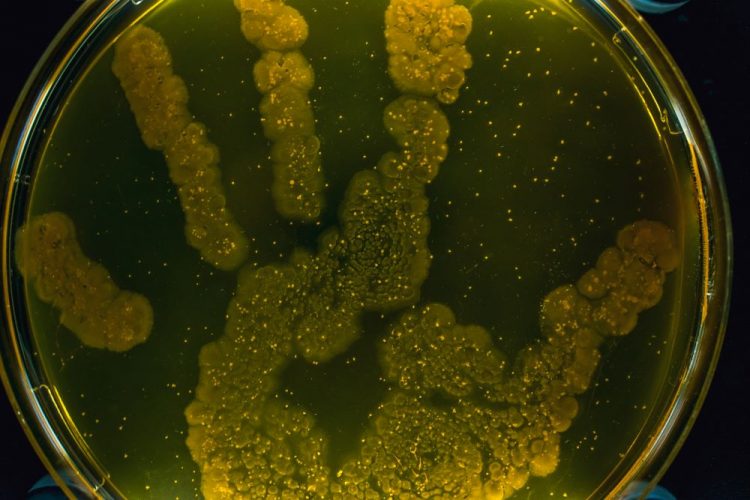
Glove prints are the most effective microbiological monitoring method during aseptic handling, finds study
Researchers evaluate the various microbiological monitoring (MM) methods employed inside a laminar airflow or safety cabinet in hospital pharmacies.