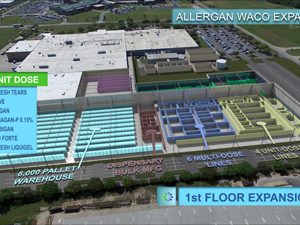
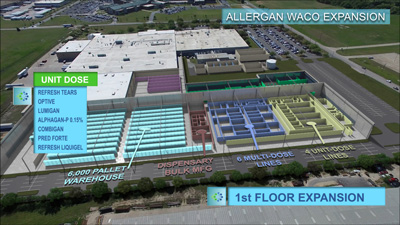
Pfizer breaks ground on new biologics manufacturing facility
17 June 2016 | By Victoria White, Digital Content Producer
Pfizer will invest more than $200 million in the development of the state-of-the-art facility that will enable the production of high-quality, complex biologics...