Packaging security trends in the pharmaceutical industry
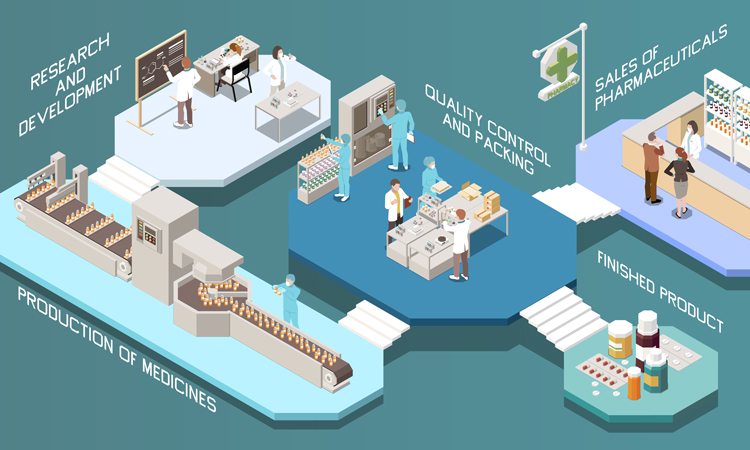
Drug safety is a huge concern for big pharma and tampering and counterfeiting in the market is dangerous for both consumers and brands. In this article, Prakash Shetty shares the latest innovations in packaging design and highlights how tamper proofing and developments in technology can protect all concerned.