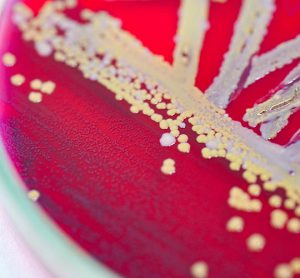
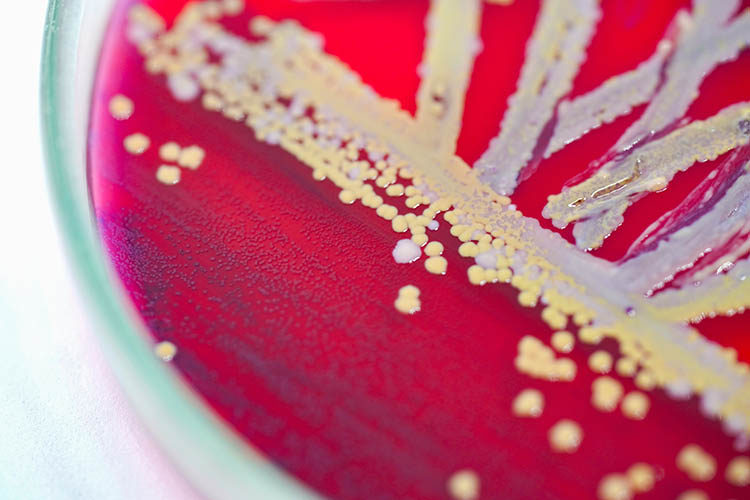
Infrared thermography can detect pre-visual bacterial growth, shows study
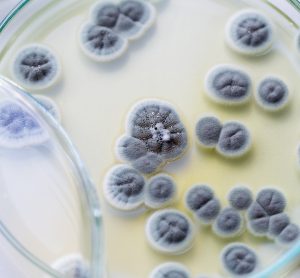
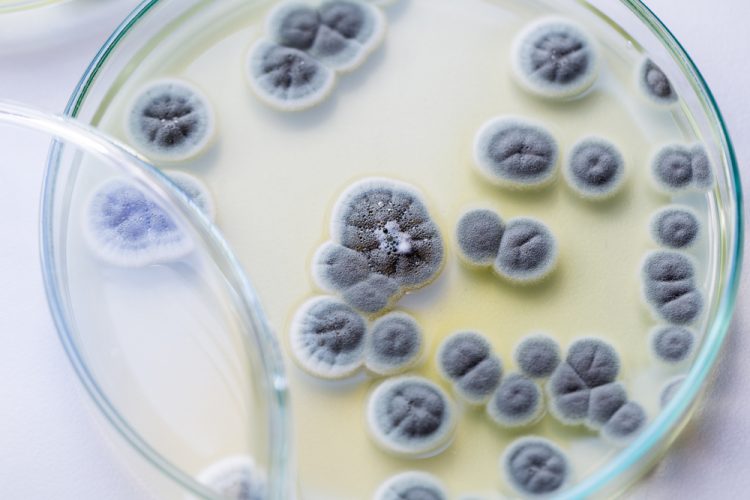
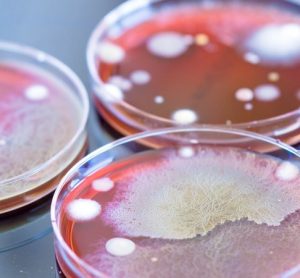
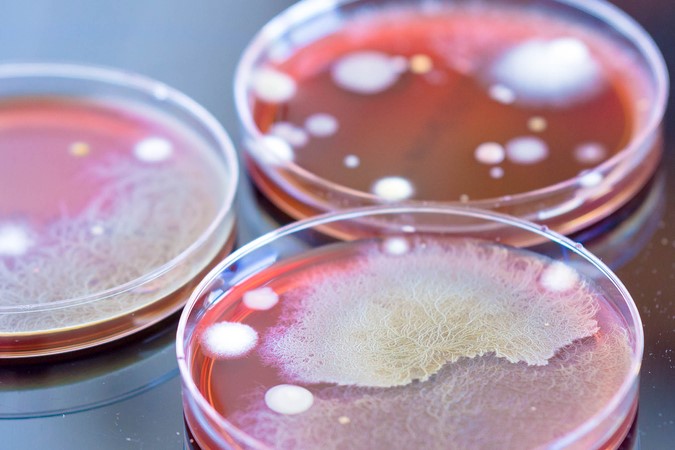
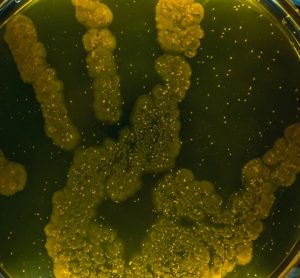
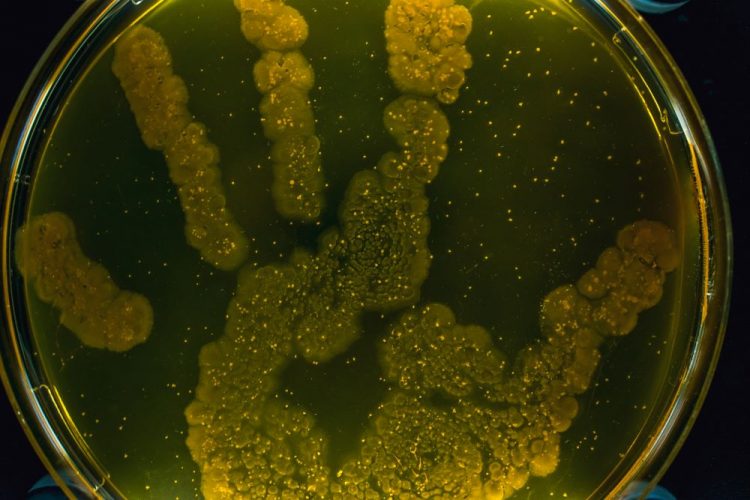
A new paper shows thermal imaging (infrared thermography) can detect E. coli and S. aureus bacteria after just six hours of incubation, long before it is visible to the human eye.

















![Scientist using scanner to read barcode - MODA-EM™ Paperless Solution [© Lonza, Basel]](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Lonza-MODA-Solution-300x278.jpg)
![Scientist using scanner to read barcode - MODA-EM™ Paperless Solution [© Lonza, Basel]](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Lonza-MODA-Solution-e1607446278766.jpg)