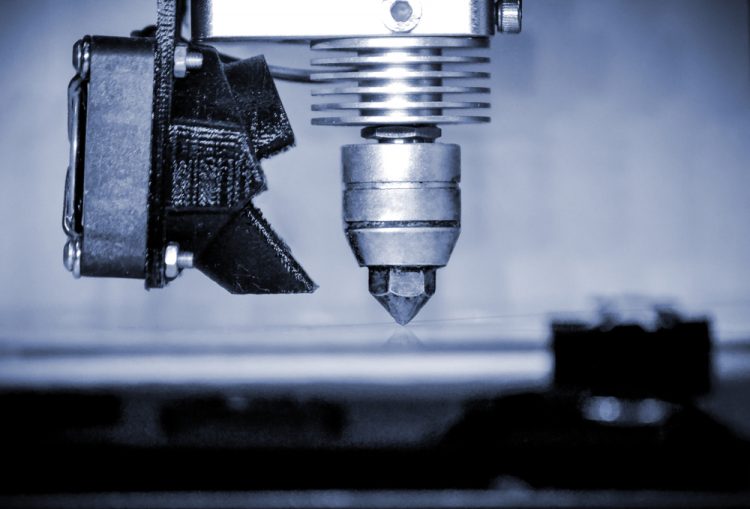
3D printing – current pharmaceutical applications and future directions
3D printing has the potential to revolutionise the pharmaceutical manufacturing industry; however, few 3D-printed products have been approved since the first in 2015. In this article, EPR’s Hannah Balfour explores the technologies currently being evaluated for use in the 3D printing of pharmaceuticals, and the work of key market players…