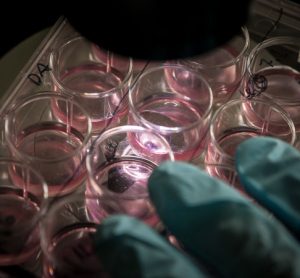
Expecting the unexpected in drug formulations
The increasing complexity of formulations and active biological products raises new challenges for pre-filled syringe development. James Mellman, Device Manager at Novartis, speaks to Nikki Withers about the challenges of selecting the right primary packaging for injectable formulations and how he has learnt to expect the unexpected.