List view / Grid view
Drug Manufacturing
EMA grants marketing authorisation for Zubsolv for opioid dependence
The EMA has granted a Marketing Authorization for Zubsolv, a novel rapidly-disintegrating treatment option for opioid dependence...
EMA grants marketing authorisation for subcutaneous formulation of Benlysta against lupus
The EU has approved a new subcutaneous formulation of Benlysta, as an add-on therapy in adult patients with active autoantibody-positive systemic lupus erythematosus...
FDA approves Vraylar in the maintenance treatment of schizophrenia
The FDA has approved the supplemental New Drug Application for Vraylarfor the maintenance treatment of adults with schizophrenia...
Vetter expands its footprint in Asia Pacific region
Company establishes its presence in South Korea...
Lab Innovations 2017 celebrates another triumph
At 4:00 pm on Thursday the 2nd of November, Lab Innovations celebrated the end of another extremely successful show at the NEC, Birmingham, UK, involving over 130 exhibitors and over 2,500 visitors...
Whitepaper: Key to outsourcing method development and validation: A pragmatic approach
In an industry that is seeing an increasing level of work being outsourced, the Contract Research Organisation (CRO) of choice needs to have proven experience in both the pragmatism and flexibility of the method developer’s mind set and a regulatory background in validation...
FDA approves Calquence for adults with mantle cell lymphoma
The FDA has granted accelerated approval to Calquence for the treatment of adults with mantle cell lymphoma...
Guide to Outsourcing
Welcome to European Pharmaceutical Review’s Guide to Outsourcing, the fourth in our series of ‘Guide to…’ supplements. In this edition, five leading companies that provide outsourcing services explain how their portfolio meets current industry needs.
Oral semaglutide shows promise for type 2 diabetes
Researchers have identified that the drug semaglutide resulted in better glycemic control than placebo for patients with type 2 diabetes...
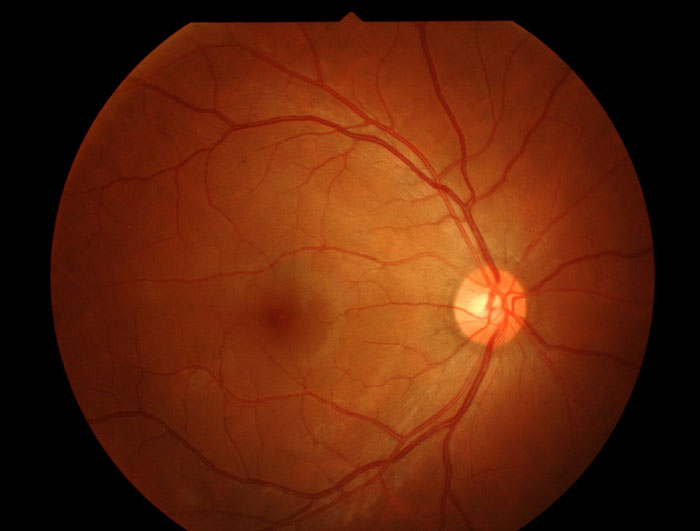
ANSM grants authorisation for phase I/II clinical study for retinitis pigmentosa
The French National Agency for Medicines and Health Products Safety is set to launch a Phase I/II clinical trial for HORA-PDE6B, in the treatment of a retinitis pigmentosa...
FDA grants breakthrough therapy designation to Tagrisso for NSCLC
The FDA has granted Breakthrough Therapy Designation for Tagrisso for the 1st-line treatment of patients with NSCLC...
NICE recommends access to venclyxto for adult leukaemia
NICE has issued a final appraisal determination recommending that venclyxto is made available to patients with chronic lymphocytic leukaemia...
Datwyler’s future-proof health care strategy
• Datwyler Sealing Solutions presents the company’s new health care offering at CPhI Worldwide • First Line – Manufacturing concept for high-quality elastomer components