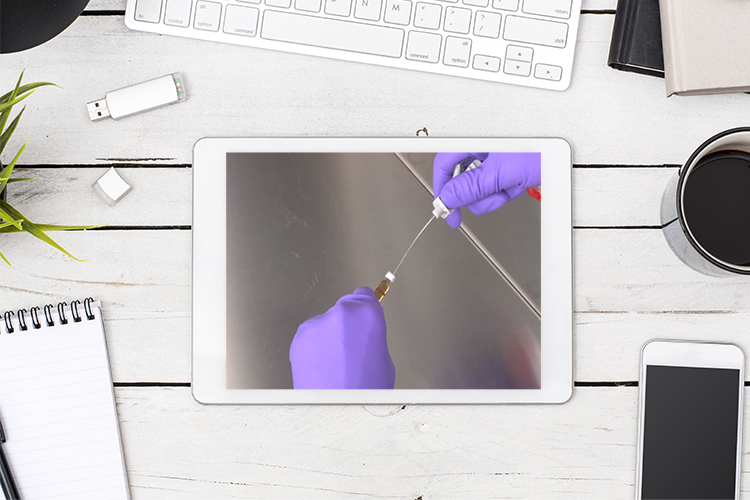
Technique to stabilise freeze-dried cellular machinery could improve cell-free biotech
Researchers have discovered low-cost preservatives that enable freeze-dried cellular machinery to retain full activity when stored at room temperature for cell-free biotechnology.