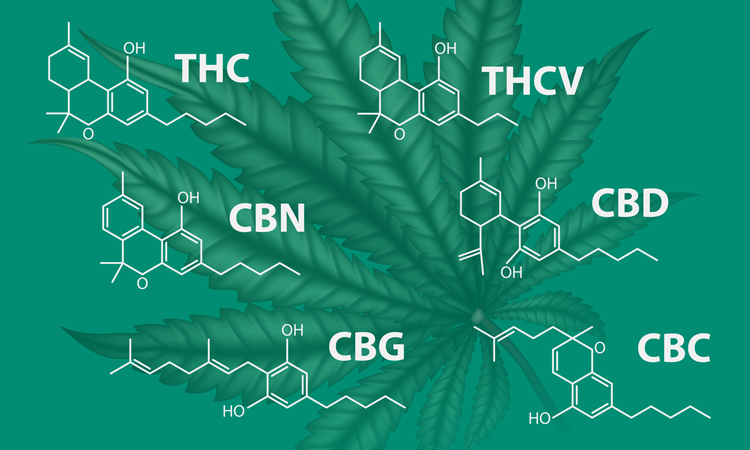
Application note: A CMC Development Decision Support Tool for Batch Genealogy Management
Successful completion of product development for active pharmaceutical ingredients (APIs) is a tremendously challenging task, in large part due to the amount of analytical data collected.