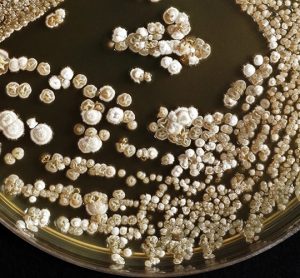
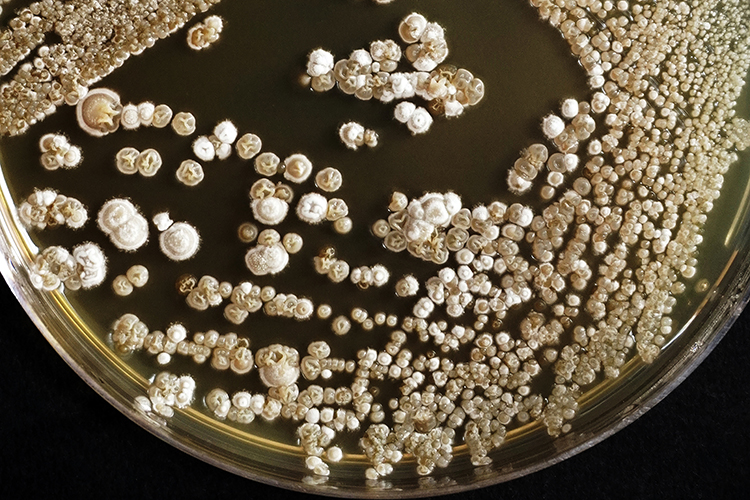
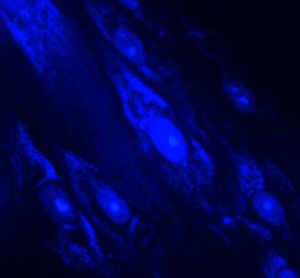
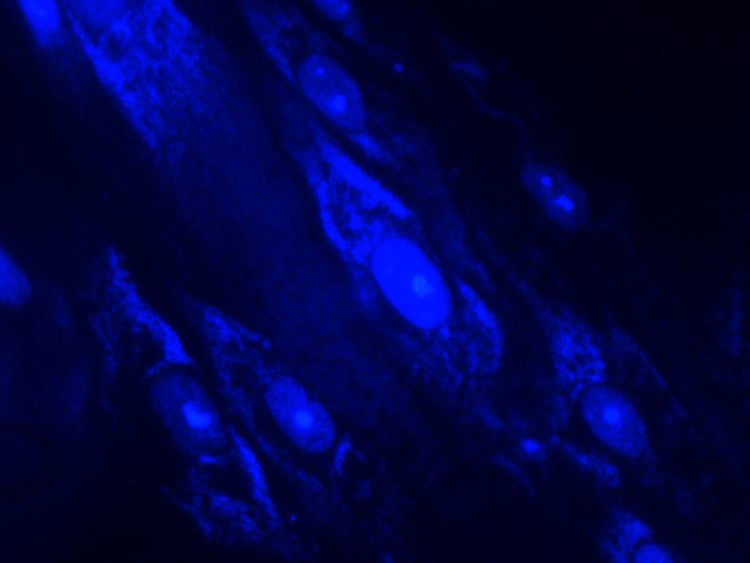
Efficient shutdown recovery: leveraging RMM to unlock value and ensure environmental quality
New technologies are awaiting implementation by the pharmaceutical industry to improve and optimise processes, but as they are not compendial and may require lengthy approval processes, their adoption as a replacement for a compendial method is slow, if at all possible. Though the industry is still contemplating how to unlock…