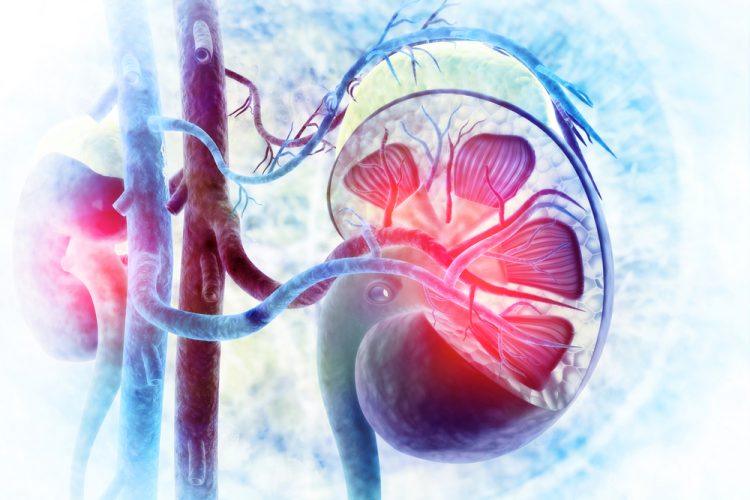
O-glycan analysis of therapeutic proteins enabled by O-glycoprotease
Glycosylation of therapeutic proteins is important to biologic drug development and is a critical quality attribute that is monitored during manufacturing. Analysis of O-glycans is technically challenging compared to that of N-glycans. In this review, Xiaofeng Shi, Saulius Vainauskas and Christopher H Taron summarise current O-glycan analytical approaches, describe the…




















![Vials labelled 'COVID-19 Vaccine Pfizer' [Giovanni Cancemi / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Pfizer-covid-19-vaccine-300x278.jpg)
![Vials labelled 'COVID-19 Vaccine Pfizer' [Giovanni Cancemi / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Pfizer-covid-19-vaccine-scaled-e1607955047519.jpg)

